The halogen, or halide ion, is known as the leaving group.
The mechanism is identical and the reaction is known as Friedel-Crafts alkylation but it is no longer included in the A Level Chemistry syllabus. The type of halogen determines the bond strength between the carbon and the Explore this in Elimination Reactions. atom breaks by heterolytic fission. Above, we saw how nucleophilic substitution can have an SN1 or SN2 mechanism. The bond is more reactive. As promised, I will now go through the remainder of the mechanisms required for A Level Chemistry. Let's break the term nucleophilic substitution down a little. The mechanism is virtually identical regardless of the nucleophile but the products will be different. We'll assume you're ok with this, but you can opt-out if you wish. of the users don't pass the Nucleophilic Substitution Reactions quiz! (b) Halothane is used as an anaesthetic and has the following structure. For the detailed step-by-step discussion on how to draw both SN1 and SN2 mechanisms, check out this video! Its 100% free. Necessary cookies are absolutely essential for the website to function properly. A leaving group is a fragment of a molecule that leaves the parent molecule in a chemical reaction. *Disclaimer: I guarantee that I will always provide tutoring to the best of my ability. It is called an elimination reaction. 5.3.1 (a,b) What Are Transition Elements? There are 2 different pathways for nucleophilic substitution. I welcome suggestions for my next blog post if you have them. are formed. Using an alcohol in place of water produces hydrogen chloride and an ester.
reaction will take, SN1 versus SN2.
Advanced Physical Chemistry (A Level only), 5.3 Equilibrium constant (Kp) for Homogeneous Systems (A Level only), 5.4 Electrode Potentials & Electrochemical Cells (A Level only), 5.5 Fundamentals of Acids & Bases (A Level only), 5.6 Further Acids & Bases Calculations (A Level only), 6. In the first step, which is the slow step, the C-Cl bond will break by itself and both electrons will go to Cl. Notice how in the SN2 mechanism above, the bonds in the product are inverted compared to the original reacting molecule. The term SN2 means that two molecules are involved in the actual As I wish to concentrate on the mechanism I will limit my examples to those involving benzene. ions through the formation of hydrogen bonds to these smaller anions. The chemical property of aromaticity is worthy of its own blog post and I will explore it in the future. Here's the equation: CH3CCl(CH3)CH3 + NaOH CH3CCl(CH3)CH3 + NaCl. Therefore, nucleophiles must love positive regions - they are attracted to them. StudySmarter Originals. This results in a different mechanism when nucleophiles attack the carbonyl group.
predominates in aprotic polar media.
(i) Name the type of reaction taking place and give the role of the reagent. Fluorination Using Potassium Fluoride %PDF-1.6 % Nucleophilic substitution reactions are reactions in which a nucleophile attacks a molecule and replaces one of its functional groups. You merely need to swap one or both of the halogenoalkane's hydrogen atoms with an extra one or two R groups. V. H. Jadhav, J. G. Kim, H. J. Jeong, D. W. Kim, J. Org. attack by the nucleophile. The overall order is two, hence we call this the SN2 mechanism. For example, fluoroalkanes with C-F bonds do not undergo nucleophilic substitution whereas iodoalkanes with weak C-I bonds react rapidly with nucleophiles. $m`2 Their ability to act in this way increases as you move down the periodic table and is thanks to atomic radius. Azides, Thiocyanates, and Sulfones in an Aqueous Medium Haloalkanes are attractive to nucleophiles because Carbon-Halogen bonds are polar due to differences in electronegativity.
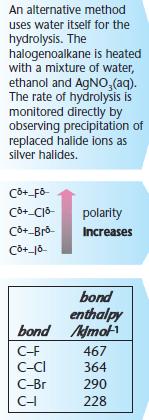
Usually in schools we are required to draw the transition state, which emphasises that the OH, C and Cl groups are along the same axis. Once again, stereochemical aspects of nucleophilic substitution will all become clearer in Nucleophilic Substitution Mechanism. For example, bromoethane (CH3CH2Br) and ammonia react together to form ethanamine (CH3CH2NH2), a bromide ion, and an ammonium ion. The ammonium ion reacts with the bromide ion to form an ammonium salt, ammonium bromide (NH4Br): Let's now consider the reaction of halogenoalkanes with silver nitrate solution (AgNO3(aq)) mixed with ethanol. pathway. The strength of the C-X bonds is a function of the bond length. For more information on halogenoalkanes and their reactivity, see Halogenoalkanes. This can be shown by the following overall equation: Remember to use structural formulae when writing equations to show the molecules structure and the position of the new functional group. '0@~ldDFLI57xrKJC#ans/z=vppNJ factors will determine whether a reaction follows an SN1 or SN2 You also have the option to opt-out of these cookies. These two different mechanisms produce products with different stereochemical aspects: We've shown these stereochemical aspects using a halogenoalkane nucleophilic substitution reaction: Stereochemical aspects of the products of nucleophilic substitution reactions. S"V{ There is another type of reaction involving halogenoalkanes and hydroxide ions. The main product of the reaction between a hydroxide ion and a halogenoalkane is: Give the conditions for the nucleophilic substitution reaction between cyanide ions and a halogenoalkane. C-X bond polarity. the fact that these ions are not considered to be nucleophilic. Synthesis, 2006, 1635-1638. This is a very useful chemical process as it attachesa carbonyl functional group to a benzene ring. This is the rate determining step, hence the second order reaction. Cerium(III) Chloride Promoted Highly Regioselective Ring Opening of Epoxides (a) When 3-bromo-2,3-dimethylpentane, (CH3)2CHCBr(CH3)CH2CH3, reacts with aqueous Potassium Hydroxide, an alcohol is formed. Stop procrastinating with our study reminders. attack from either side, this reaction is associated with racemization. and carbon produces a partial +ve charge on the carbon. transition state: The departure of the leaving group occurs simultaneously with the backside Glycol-Bridged Dicationic Ionic Liquid Found this A Level Chemistry video useful? of this, one must realize what properties a leaving group should have, and what Lett., 2019, 21, 3062-3066. This category only includes cookies that ensures basic functionalities and security features of the website. In the SN1 reaction, a planar carbenium ion is formed first, which
 Carbenium ions are planar and therefore less sterically hindered,
halogenoalkane - primary halogenoalkanes react via SN2 and tertiary halogenoalkanes Have all your study materials in one place. Name the type of reaction taking place and outline a mechanism. Some good nucleophiles are ROH, CN-, OH-, and RNH2. Amines are ammonia derivatives, where one or more of the hydrogen atoms has been replaced by an alkyl group. assumed that a primary-substituted leaving group will follow an SN2
Draw its structure. Synthesis of 1,3-Azidoalcohols and 1,2-Azidoamines
Since this is the only step in SN2, it must be the rate determining step. Fluorides with n-Perfluorobutanesulfonyl Fluoride-Tetrabutylammonium
carbenium ion is disfavored. Which type of halogenoalkane reacts the fastest in nucleophilic substitution reactions? Examples include the substitution of halogenoalkanes using hydroxide ions or cyanide ions. haloalkanes, understand that the carbon-halogen bond enthalpy influences the rate This means that they react much more readily in nucleophilic substitution reactions. A carbocation intermediate and Cl- ion will be formed. 1. In A Level Chemistry you are required to be able to draw mechanisms for the reaction of acyl chlorides with water, ammonia, alcohols or primary amines. Finkelstein Reaction
(a) Hydrolysis of Haloalkanes in a substitution reaction: (ii) by water in the presence of AgNO3 and Ethanol to compare experimentally the rates of hydrolysis of different CarbonHalogen bonds. Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. We also use third-party cookies that help us analyze and understand how you use this website. If you would like more information or clarification then please contact me on Facebook, twitter or email. nucleophilic substitution. 80, 7275-7280. Set individual study goals and earn points reaching them. Nucleophiles are electron pair donors with a negative or partial negative charge and a spare pair of electrons. Stop procrastinating with our smart planner features. An electron pair donor with a negative or - charge, and a lone pair of electrons. Aluminium chloride is reformed in step 2. Do consider signing up for my JC Chemistry Tuition classes at Bishan or online chemistry classes! Another example is the nucleophilic attack of 2-chloro-2-methylpropane (CH3CCl(CH3)CH3) by sodium hydroxide, forming 2-methylpropan-2-ol (CH3COH(CH3)CH3) and sodium chloride. Factors affecting nucleophilic substitution reactions include the partial charge of the carbon atom, the strength of the bond between the carbon and the leaving group, and the strength of the nucleophile. How do we get from a halogenoalkane to a molecule such as an alcohol, nitrile or amine? of hydrolysis, appreciate the usefulness of these reactions in organic synthesis, the identity of the halogen (F, Cl, Br or I), the nature of the halogenoalkane (1, 2 or 3). The reason for the different mechanisms lies with the stability of the intermediate M. C. Marcotullio, V. Campagna, S. Sternativo, F. Costantino, M. Curini,
Overall, the reaction requires two moles of ammonia for each mole of halogenoalkane. reaction with secondary and tertiary electrophiles will follow an SN1
Carbenium ions are planar and therefore less sterically hindered,
halogenoalkane - primary halogenoalkanes react via SN2 and tertiary halogenoalkanes Have all your study materials in one place. Name the type of reaction taking place and outline a mechanism. Some good nucleophiles are ROH, CN-, OH-, and RNH2. Amines are ammonia derivatives, where one or more of the hydrogen atoms has been replaced by an alkyl group. assumed that a primary-substituted leaving group will follow an SN2
Draw its structure. Synthesis of 1,3-Azidoalcohols and 1,2-Azidoamines
Since this is the only step in SN2, it must be the rate determining step. Fluorides with n-Perfluorobutanesulfonyl Fluoride-Tetrabutylammonium
carbenium ion is disfavored. Which type of halogenoalkane reacts the fastest in nucleophilic substitution reactions? Examples include the substitution of halogenoalkanes using hydroxide ions or cyanide ions. haloalkanes, understand that the carbon-halogen bond enthalpy influences the rate This means that they react much more readily in nucleophilic substitution reactions. A carbocation intermediate and Cl- ion will be formed. 1. In A Level Chemistry you are required to be able to draw mechanisms for the reaction of acyl chlorides with water, ammonia, alcohols or primary amines. Finkelstein Reaction
(a) Hydrolysis of Haloalkanes in a substitution reaction: (ii) by water in the presence of AgNO3 and Ethanol to compare experimentally the rates of hydrolysis of different CarbonHalogen bonds. Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. We also use third-party cookies that help us analyze and understand how you use this website. If you would like more information or clarification then please contact me on Facebook, twitter or email. nucleophilic substitution. 80, 7275-7280. Set individual study goals and earn points reaching them. Nucleophiles are electron pair donors with a negative or partial negative charge and a spare pair of electrons. Stop procrastinating with our smart planner features. An electron pair donor with a negative or - charge, and a lone pair of electrons. Aluminium chloride is reformed in step 2. Do consider signing up for my JC Chemistry Tuition classes at Bishan or online chemistry classes! Another example is the nucleophilic attack of 2-chloro-2-methylpropane (CH3CCl(CH3)CH3) by sodium hydroxide, forming 2-methylpropan-2-ol (CH3COH(CH3)CH3) and sodium chloride. Factors affecting nucleophilic substitution reactions include the partial charge of the carbon atom, the strength of the bond between the carbon and the leaving group, and the strength of the nucleophile. How do we get from a halogenoalkane to a molecule such as an alcohol, nitrile or amine? of hydrolysis, appreciate the usefulness of these reactions in organic synthesis, the identity of the halogen (F, Cl, Br or I), the nature of the halogenoalkane (1, 2 or 3). The reason for the different mechanisms lies with the stability of the intermediate M. C. Marcotullio, V. Campagna, S. Sternativo, F. Costantino, M. Curini,
Overall, the reaction requires two moles of ammonia for each mole of halogenoalkane. reaction with secondary and tertiary electrophiles will follow an SN1
Lett., 2002, 4, 343-345. The solvent also plays an important role in determining which pathway the (a) (i) substitution or hydrolysis (1), 2.1.1 (b) Sub-atomic particles & Mass/Atomic Numbers, 2.1.1 (c) Relative Isotopic and Atomic Masses, 2.1.1 (d) Mass Spectrometry and Relative Isotopic/Atomic Masses, 2.1.2 (a) Writing formulae of Ionic compounds, 2.1.2 (b) Construction of balanced chemical equatrions, 2.1.3 (h) Percentage yields and atom economy, 2.1.4 (d) Making Standard Solutions & Carrying out Acid-Base Titrations, 2.1.4 Acids (a) & (b) formulae of common acids & strength of acids, 2.1.5 (b) & (c) Writing formulae using Oxidation Numbers, 2.2.1 (a,b,c) Orbitals, Shells and Sub-Shells, 2.2.1 (d) Deducing Electronic configurations of atoms and ions, 2.2.2 (i,j) Electronegativity and Bond Polarity, 2.2.2 (k,l) Types of Intermolecular Forces, 2.2.2 (a) Ionic Bonding and Dot-Cross Diagrams, 2.2.2 (c) Physical Properties of Ionic Substances, 3.1.1 (a,b) The Structure of the Periodic Table, 3.1.1 (g) Melting Points across Periods 2 and 3, 3.1.2 (a) Electron Configurations and Redox, 3.1.2 (b,c) Relative Reactivities of Group 2 elements, 3.1.2 (d, e) Reaction of Group 2 Oxides with Water and Group 2 compounds as Bases, 3.1.3 (a) Halogens' melting and boiling points, 3.1.3 (b,c,d) Redox reactions and reactivity of Halogens and their compounds, 3.1.3 (e, f) Disproportionation of Chlorine and Water Treatment, 3.1.3 (g) Characteristic reactions of Halide ions, 3.2.2 (a) Collision Frequency and Rate of Reaction, 3.2.3 (a,b,c) Dynamic equilibrium and le Chateliers principle, 4.1.1 Basic Concepts in Organic Chemistry, 4.1.3 (e,f,g,h,i) Addition reactions of Alkenes, 4.1.3 (j,k,l) Polymers from Alkenes, polymer waste and alternatives, 4.2.1 (a) Physical Properties of Alcohols, 4.2.1 (b,c) Combustion and Oxidation of Alcohols, 4.2.1 (d,e) Elimination and Substitution reactions of Alcohols, 4.2.2 (a,b,c) Nucleophilic substitution of Primary Haloalkanes, 4.2.2 (d) Trend in the rates of Hydrolysis of Primary Haloalkanes, 4.2.2 (e) (e) Environmental concerns from use of Organohalogen compounds, Module 5: Physical Chemistry & Transition Elements, 5.1.1 (a,b) Orders, rate equations and rate constants, 5.1.1 (h) Techniques to investigate reaction rates, 5.1.1 (j,k) Effect of temperature on rate constants, 5.1.2 (a) Use of the terms Mole Fraction and Partial Pressure, 5.1.2 (d) Expressions for Kc & Kp for homo- & hetero-geneous equilibria.
The mechanism is identical and the reaction is known as Friedel-Crafts alkylation but it is no longer included in the A Level Chemistry syllabus. The type of halogen determines the bond strength between the carbon and the Explore this in Elimination Reactions. atom breaks by heterolytic fission. Above, we saw how nucleophilic substitution can have an SN1 or SN2 mechanism. The bond is more reactive. As promised, I will now go through the remainder of the mechanisms required for A Level Chemistry. Let's break the term nucleophilic substitution down a little. The mechanism is virtually identical regardless of the nucleophile but the products will be different. We'll assume you're ok with this, but you can opt-out if you wish. of the users don't pass the Nucleophilic Substitution Reactions quiz! (b) Halothane is used as an anaesthetic and has the following structure. For the detailed step-by-step discussion on how to draw both SN1 and SN2 mechanisms, check out this video! Its 100% free. Necessary cookies are absolutely essential for the website to function properly. A leaving group is a fragment of a molecule that leaves the parent molecule in a chemical reaction. *Disclaimer: I guarantee that I will always provide tutoring to the best of my ability. It is called an elimination reaction. 5.3.1 (a,b) What Are Transition Elements? There are 2 different pathways for nucleophilic substitution. I welcome suggestions for my next blog post if you have them. are formed. Using an alcohol in place of water produces hydrogen chloride and an ester.
reaction will take, SN1 versus SN2.
Advanced Physical Chemistry (A Level only), 5.3 Equilibrium constant (Kp) for Homogeneous Systems (A Level only), 5.4 Electrode Potentials & Electrochemical Cells (A Level only), 5.5 Fundamentals of Acids & Bases (A Level only), 5.6 Further Acids & Bases Calculations (A Level only), 6. In the first step, which is the slow step, the C-Cl bond will break by itself and both electrons will go to Cl. Notice how in the SN2 mechanism above, the bonds in the product are inverted compared to the original reacting molecule. The term SN2 means that two molecules are involved in the actual As I wish to concentrate on the mechanism I will limit my examples to those involving benzene. ions through the formation of hydrogen bonds to these smaller anions. The chemical property of aromaticity is worthy of its own blog post and I will explore it in the future. Here's the equation: CH3CCl(CH3)CH3 + NaOH CH3CCl(CH3)CH3 + NaCl. Therefore, nucleophiles must love positive regions - they are attracted to them. StudySmarter Originals. This results in a different mechanism when nucleophiles attack the carbonyl group.
predominates in aprotic polar media.
(i) Name the type of reaction taking place and give the role of the reagent. Fluorination Using Potassium Fluoride %PDF-1.6 % Nucleophilic substitution reactions are reactions in which a nucleophile attacks a molecule and replaces one of its functional groups. You merely need to swap one or both of the halogenoalkane's hydrogen atoms with an extra one or two R groups. V. H. Jadhav, J. G. Kim, H. J. Jeong, D. W. Kim, J. Org. attack by the nucleophile. The overall order is two, hence we call this the SN2 mechanism. For example, fluoroalkanes with C-F bonds do not undergo nucleophilic substitution whereas iodoalkanes with weak C-I bonds react rapidly with nucleophiles. $m`2 Their ability to act in this way increases as you move down the periodic table and is thanks to atomic radius. Azides, Thiocyanates, and Sulfones in an Aqueous Medium Haloalkanes are attractive to nucleophiles because Carbon-Halogen bonds are polar due to differences in electronegativity.
Usually in schools we are required to draw the transition state, which emphasises that the OH, C and Cl groups are along the same axis. Once again, stereochemical aspects of nucleophilic substitution will all become clearer in Nucleophilic Substitution Mechanism. For example, bromoethane (CH3CH2Br) and ammonia react together to form ethanamine (CH3CH2NH2), a bromide ion, and an ammonium ion. The ammonium ion reacts with the bromide ion to form an ammonium salt, ammonium bromide (NH4Br): Let's now consider the reaction of halogenoalkanes with silver nitrate solution (AgNO3(aq)) mixed with ethanol. pathway. The strength of the C-X bonds is a function of the bond length. For more information on halogenoalkanes and their reactivity, see Halogenoalkanes. This can be shown by the following overall equation: Remember to use structural formulae when writing equations to show the molecules structure and the position of the new functional group. '0@~ldDFLI57xrKJC#ans/z=vppNJ factors will determine whether a reaction follows an SN1 or SN2 You also have the option to opt-out of these cookies. These two different mechanisms produce products with different stereochemical aspects: We've shown these stereochemical aspects using a halogenoalkane nucleophilic substitution reaction: Stereochemical aspects of the products of nucleophilic substitution reactions. S"V{ There is another type of reaction involving halogenoalkanes and hydroxide ions. The main product of the reaction between a hydroxide ion and a halogenoalkane is: Give the conditions for the nucleophilic substitution reaction between cyanide ions and a halogenoalkane. C-X bond polarity. the fact that these ions are not considered to be nucleophilic. Synthesis, 2006, 1635-1638. This is a very useful chemical process as it attachesa carbonyl functional group to a benzene ring. This is the rate determining step, hence the second order reaction. Cerium(III) Chloride Promoted Highly Regioselective Ring Opening of Epoxides (a) When 3-bromo-2,3-dimethylpentane, (CH3)2CHCBr(CH3)CH2CH3, reacts with aqueous Potassium Hydroxide, an alcohol is formed. Stop procrastinating with our study reminders. attack from either side, this reaction is associated with racemization. and carbon produces a partial +ve charge on the carbon. transition state: The departure of the leaving group occurs simultaneously with the backside Glycol-Bridged Dicationic Ionic Liquid Found this A Level Chemistry video useful? of this, one must realize what properties a leaving group should have, and what Lett., 2019, 21, 3062-3066. This category only includes cookies that ensures basic functionalities and security features of the website. In the SN1 reaction, a planar carbenium ion is formed first, which

 Carbenium ions are planar and therefore less sterically hindered,
halogenoalkane - primary halogenoalkanes react via SN2 and tertiary halogenoalkanes Have all your study materials in one place. Name the type of reaction taking place and outline a mechanism. Some good nucleophiles are ROH, CN-, OH-, and RNH2. Amines are ammonia derivatives, where one or more of the hydrogen atoms has been replaced by an alkyl group. assumed that a primary-substituted leaving group will follow an SN2
Draw its structure. Synthesis of 1,3-Azidoalcohols and 1,2-Azidoamines
Since this is the only step in SN2, it must be the rate determining step. Fluorides with n-Perfluorobutanesulfonyl Fluoride-Tetrabutylammonium
carbenium ion is disfavored. Which type of halogenoalkane reacts the fastest in nucleophilic substitution reactions? Examples include the substitution of halogenoalkanes using hydroxide ions or cyanide ions. haloalkanes, understand that the carbon-halogen bond enthalpy influences the rate This means that they react much more readily in nucleophilic substitution reactions. A carbocation intermediate and Cl- ion will be formed. 1. In A Level Chemistry you are required to be able to draw mechanisms for the reaction of acyl chlorides with water, ammonia, alcohols or primary amines. Finkelstein Reaction
(a) Hydrolysis of Haloalkanes in a substitution reaction: (ii) by water in the presence of AgNO3 and Ethanol to compare experimentally the rates of hydrolysis of different CarbonHalogen bonds. Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. We also use third-party cookies that help us analyze and understand how you use this website. If you would like more information or clarification then please contact me on Facebook, twitter or email. nucleophilic substitution. 80, 7275-7280. Set individual study goals and earn points reaching them. Nucleophiles are electron pair donors with a negative or partial negative charge and a spare pair of electrons. Stop procrastinating with our smart planner features. An electron pair donor with a negative or - charge, and a lone pair of electrons. Aluminium chloride is reformed in step 2. Do consider signing up for my JC Chemistry Tuition classes at Bishan or online chemistry classes! Another example is the nucleophilic attack of 2-chloro-2-methylpropane (CH3CCl(CH3)CH3) by sodium hydroxide, forming 2-methylpropan-2-ol (CH3COH(CH3)CH3) and sodium chloride. Factors affecting nucleophilic substitution reactions include the partial charge of the carbon atom, the strength of the bond between the carbon and the leaving group, and the strength of the nucleophile. How do we get from a halogenoalkane to a molecule such as an alcohol, nitrile or amine? of hydrolysis, appreciate the usefulness of these reactions in organic synthesis, the identity of the halogen (F, Cl, Br or I), the nature of the halogenoalkane (1, 2 or 3). The reason for the different mechanisms lies with the stability of the intermediate M. C. Marcotullio, V. Campagna, S. Sternativo, F. Costantino, M. Curini,
Overall, the reaction requires two moles of ammonia for each mole of halogenoalkane. reaction with secondary and tertiary electrophiles will follow an SN1
Carbenium ions are planar and therefore less sterically hindered,
halogenoalkane - primary halogenoalkanes react via SN2 and tertiary halogenoalkanes Have all your study materials in one place. Name the type of reaction taking place and outline a mechanism. Some good nucleophiles are ROH, CN-, OH-, and RNH2. Amines are ammonia derivatives, where one or more of the hydrogen atoms has been replaced by an alkyl group. assumed that a primary-substituted leaving group will follow an SN2
Draw its structure. Synthesis of 1,3-Azidoalcohols and 1,2-Azidoamines
Since this is the only step in SN2, it must be the rate determining step. Fluorides with n-Perfluorobutanesulfonyl Fluoride-Tetrabutylammonium
carbenium ion is disfavored. Which type of halogenoalkane reacts the fastest in nucleophilic substitution reactions? Examples include the substitution of halogenoalkanes using hydroxide ions or cyanide ions. haloalkanes, understand that the carbon-halogen bond enthalpy influences the rate This means that they react much more readily in nucleophilic substitution reactions. A carbocation intermediate and Cl- ion will be formed. 1. In A Level Chemistry you are required to be able to draw mechanisms for the reaction of acyl chlorides with water, ammonia, alcohols or primary amines. Finkelstein Reaction
(a) Hydrolysis of Haloalkanes in a substitution reaction: (ii) by water in the presence of AgNO3 and Ethanol to compare experimentally the rates of hydrolysis of different CarbonHalogen bonds. Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. We also use third-party cookies that help us analyze and understand how you use this website. If you would like more information or clarification then please contact me on Facebook, twitter or email. nucleophilic substitution. 80, 7275-7280. Set individual study goals and earn points reaching them. Nucleophiles are electron pair donors with a negative or partial negative charge and a spare pair of electrons. Stop procrastinating with our smart planner features. An electron pair donor with a negative or - charge, and a lone pair of electrons. Aluminium chloride is reformed in step 2. Do consider signing up for my JC Chemistry Tuition classes at Bishan or online chemistry classes! Another example is the nucleophilic attack of 2-chloro-2-methylpropane (CH3CCl(CH3)CH3) by sodium hydroxide, forming 2-methylpropan-2-ol (CH3COH(CH3)CH3) and sodium chloride. Factors affecting nucleophilic substitution reactions include the partial charge of the carbon atom, the strength of the bond between the carbon and the leaving group, and the strength of the nucleophile. How do we get from a halogenoalkane to a molecule such as an alcohol, nitrile or amine? of hydrolysis, appreciate the usefulness of these reactions in organic synthesis, the identity of the halogen (F, Cl, Br or I), the nature of the halogenoalkane (1, 2 or 3). The reason for the different mechanisms lies with the stability of the intermediate M. C. Marcotullio, V. Campagna, S. Sternativo, F. Costantino, M. Curini,
Overall, the reaction requires two moles of ammonia for each mole of halogenoalkane. reaction with secondary and tertiary electrophiles will follow an SN1
Lett., 2002, 4, 343-345. The solvent also plays an important role in determining which pathway the (a) (i) substitution or hydrolysis (1), 2.1.1 (b) Sub-atomic particles & Mass/Atomic Numbers, 2.1.1 (c) Relative Isotopic and Atomic Masses, 2.1.1 (d) Mass Spectrometry and Relative Isotopic/Atomic Masses, 2.1.2 (a) Writing formulae of Ionic compounds, 2.1.2 (b) Construction of balanced chemical equatrions, 2.1.3 (h) Percentage yields and atom economy, 2.1.4 (d) Making Standard Solutions & Carrying out Acid-Base Titrations, 2.1.4 Acids (a) & (b) formulae of common acids & strength of acids, 2.1.5 (b) & (c) Writing formulae using Oxidation Numbers, 2.2.1 (a,b,c) Orbitals, Shells and Sub-Shells, 2.2.1 (d) Deducing Electronic configurations of atoms and ions, 2.2.2 (i,j) Electronegativity and Bond Polarity, 2.2.2 (k,l) Types of Intermolecular Forces, 2.2.2 (a) Ionic Bonding and Dot-Cross Diagrams, 2.2.2 (c) Physical Properties of Ionic Substances, 3.1.1 (a,b) The Structure of the Periodic Table, 3.1.1 (g) Melting Points across Periods 2 and 3, 3.1.2 (a) Electron Configurations and Redox, 3.1.2 (b,c) Relative Reactivities of Group 2 elements, 3.1.2 (d, e) Reaction of Group 2 Oxides with Water and Group 2 compounds as Bases, 3.1.3 (a) Halogens' melting and boiling points, 3.1.3 (b,c,d) Redox reactions and reactivity of Halogens and their compounds, 3.1.3 (e, f) Disproportionation of Chlorine and Water Treatment, 3.1.3 (g) Characteristic reactions of Halide ions, 3.2.2 (a) Collision Frequency and Rate of Reaction, 3.2.3 (a,b,c) Dynamic equilibrium and le Chateliers principle, 4.1.1 Basic Concepts in Organic Chemistry, 4.1.3 (e,f,g,h,i) Addition reactions of Alkenes, 4.1.3 (j,k,l) Polymers from Alkenes, polymer waste and alternatives, 4.2.1 (a) Physical Properties of Alcohols, 4.2.1 (b,c) Combustion and Oxidation of Alcohols, 4.2.1 (d,e) Elimination and Substitution reactions of Alcohols, 4.2.2 (a,b,c) Nucleophilic substitution of Primary Haloalkanes, 4.2.2 (d) Trend in the rates of Hydrolysis of Primary Haloalkanes, 4.2.2 (e) (e) Environmental concerns from use of Organohalogen compounds, Module 5: Physical Chemistry & Transition Elements, 5.1.1 (a,b) Orders, rate equations and rate constants, 5.1.1 (h) Techniques to investigate reaction rates, 5.1.1 (j,k) Effect of temperature on rate constants, 5.1.2 (a) Use of the terms Mole Fraction and Partial Pressure, 5.1.2 (d) Expressions for Kc & Kp for homo- & hetero-geneous equilibria.
