Mis-aisa-The latest News,Tech,Industry,Environment,Low Carbon,Resource,Innovations. Oxide compounds are not conductive to electricity. Vanadium is Find another reaction.
Formula Weight: 181.88: Physical Form-10 Mesh: Density: 3.357 g/mL: Chemical Name or Material: quent air-annealing under suitable conditions yields vanadium oxide bers of 2090 nm diameter and 1050 m2/g surface area. DOT ID & Guide Solubility.
Pubchem CID. In the process, the vanadium(V) oxide is reduced to vanadium(IV) oxide. Vanadium pentoxide (V 2 O 5) is the most common commercial form. Video.
Vanadium nitride is known as VN powder, it is an important vanadium alloy additive, and vanadium nitride is used in lithium electronic batteries and energy storage materials. Vanadium supplements are used as medicine.
Vanadium (V) oxide ( vanadia) is the inorganic compound with the formula V 2 O 5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Three techniques have been employed to establish the temperature dependence of the terminal solubility of oxygen in vanadium in the range 200 750 C. Vanadium. NA. Trending. The solubility controls on vanadium (V) in groundwater were studied due to concerns over possible harmful health effects of ingesting V in drinking water.
Structure, properties, spectra, suppliers and links for: Vanadium(V) oxide, Vanadium, 1314-62-1. The metal is used principally as an alloying addition to high-strength low-alloy (HSLA) steels and, to a lesser extent, in tool steels and iron and steel castings.
The solubility of the substances. Safety Information.
FOB Price: US$ 25-50 / kg. Search. 183.91 : 0.277 : a.
represent an important subclass within reducible metal oxide (RMO) materials that are used in a wide variety of applications including electrical and optical devices, smart windows, and batteries, and as heterogenous catalysts for the activation of environmental contaminants and organic substrates. Reduce with solid sodium bisulfite until the color turns BLUE. Vanadium and Its Soluble Inorganic Compounds Other Than Vanadium Pentoxide (CASRN 7440-62-2 and Others) Derivation of Subchronic and Chronic Oral RfDs . [ Check the balance ] Vanadium(V) oxide react with sulfuric acid to produce vanadyl sulfate, water and oxygen.  Vanadium Oxide is available as a 99.9 pure (metals basis) powder in batch quantities for use as a cathode material in lithion-ion batteries. Vanadium | V | CID 23990 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
Vanadium Oxide is available as a 99.9 pure (metals basis) powder in batch quantities for use as a cathode material in lithion-ion batteries. Vanadium | V | CID 23990 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
This represents the first epoxide-assisted gelation developed for vanadium oxide gels and serves as a potential economical replacement for the fabrication of vanadium oxide gels via the hydrolysis of vanadium alkoxides.
3. Flash Point. Vanadium (V) Oxide is a highly insoluble thermally stable Vanadium source suitable for glass, optic, and ceramic applications. Pigment & Dyestuff; Organic Intermediate; VANADIUM(IV) OXIDE is a blue-black solid, having a distorted rutile (TiO2) structure. Related to this ability, V 2 O 5 catalyses several useful aerobic oxidation reactions, the largest scale of which underpins the production of
Trending.
Abstract. The solubility controls on vanadium (V) in groundwater were studied due to concerns over possible harmful health effects of ingesting V in drinking water. About 80% of the vanadium produced is used as a steel additive.
It absorb oxygen in air and turn to vanadium (IV) oxide .
Slightly soluble in water.
Slightly soluble in water. Vanadium Oxide: A compound of vanadium with oxygen, for example, vanadium tetroxide (V 2 O 4), vanadium trioxide or sesquioxide (V 2 O 3), vanadium oxide (VO), and vanadium pentoxide (V 2 O 5). National Library of Medicine.
Results indicated that V may be precipitating as V 3+ - or mixed V 3+ /Fe 3+ -oxides in anoxic groundwater, which is consistent with results of a previous study. Sodium metavanadate is used as an analytical reagent and mordant.
Vanadium trioxide undergoes slow air oxidation at room temperature [Kirk-Othmer].
vanadium oxide . 0.8%. Insoluble in water. 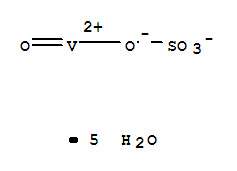 Oxidize with dilute (0.5%) HO to get GREEN.
Oxidize with dilute (0.5%) HO to get GREEN.
It is also found to be useful in the photography industry and the pharmaceutical industry. Water solubility: Water soluble Enthalpy of Vaporization: 41.82 kJ/mol Boiling Point: 105 C at 760 mmHg Vapour Pressure: 16.4 mmHg at 25C Appearance: A pale yellow liquid with an alcohol-like odor Structure of Vanadium oxide triisobutoxide (CAS NO.19120-62-8 ) : It dissolves in water to form acidic solutions and dissolves in acids. Solubility in water: 0.8g/100 ml (20C) Melting point: Melting Point::690C: Boiling point: Boiling Point::1,750C (decomposes) Structure Coordination Vanadium Oxide is an extremely toxic compound to humans.
An electrochemical cell containing a cathode comprising silver vanadium oxide and an anode comprising lithium is disclosed that includes an improved electrolyte composition having the solvents propylene carbonate and 1,2-dimethoxyethane, and an additional third solvent that reduces the solubility of the composition of the silver vanadium cathode material. Vanadium (V) Oxide is a highly insoluble thermally stable Vanadium source suitable for glass, optic and ceramic applications. Formula V2O5 Purity 99.99+% CAS Number 1314-62-1 Molecular Weight 181.88 Color & Form Orange to brown solid Boiling Point 1750 C (dec.) Melting Point 690 C Specific Gravity 3.357 Solubility in water Insoluble Download SDS (PDF) Vanadium (V) Oxide 50 g $ 485.00 Vanadium (V) Oxide 10 g $ 120.00 Add to cart Request a Quote
The solubility of oxygen in vanadium at temperatures between 200and 750 can be adequately expressed by the equation log Cg (wt.%)= 0.705-515 Q,) (-1). A silvery metal that resists corrosion.
Upper Explosive Limit. Formed phases and microstructures of saturated samples were identified with SEMEDS analysis and XRD.
Less than 1% of vanadium, and as little chromium, makes steel shock resistant and vibration resistant. Jump to main content Jump to site nav.
Brand: Todini Metal.
If it is attacked by alkali, it forms water-soluble vanadates.
National Center for Biotechnology Information. When temperature above 700, V2O5 obviously volatilize. 3.36. Order: 1 kg. Slightly soluble in water. Market Focus.
Maximum solubility of vanadium oxide was between 15 and 20% independent of basicity. Not Intended for Diagnostic or Therapeutic Use. 183.91 : 0.277 : a.
Search. Sigma-Aldrich. In addition to this, it is used in the making of vanadium alloy and vanadium catalyst.
Specific Gravity.
Divanadium pentoxide fume, Vanadic anhydride fume, Vanadium oxide fume, Vanadium pentaoxide fume CAS No. Get Contact details & address of companies manufacturing and supplying Vanadium Oxide, 1314-62-1, V2O5 across India. Vanadium concentrations in the northeastern San Joaquin Valley ranged from 25 g/L) and lowest in samples collected from anoxic groundwater (70% < 0.8 g/L). Notably, the materials from the SVPO family with the lowest vanadium solubility are Ag2VO2PO4 and Ag2VP2O8. c . Introduction Vanadium oxides (e.g. Search. 2V2O5+ 4H2SO44VOSO4+ 4H2O + O2. Others are.
FINAL . The solubility of vanadium oxide in the SiO2CaOVOX system was investigated as a function of basicity (CaO/SiO2) at a fixed temperature of 1600C and oxygen partial pressure of 1010 atm. 4VO 2 + O 2 2V 2 O 5.
Pour a little of the PURPLE solution though glass wool (to avoid getting zinc granules in) into the next flask.
vanadium v oxide, vanadium pentoxide, divanadium pentaoxide, divanadium pentoxide, vanadic anhydride, vanadin v oxide, vanadium oxide v2o5, vanadium pentaoxide, vanadic acid anhydride Solubility Information: Slightly soluble in water.
VANADIUM OXIDE VNO CAUTIONARY RESPONSE INFORMATION Common Synonyms Solid, granular or flakes Red, brown or grey Odorless Vanadium (V) oxide SOLUBILITY IN WATER Temperature (degrees F) Pounds per 100 pounds of water I N S O L U B L E 9.25 SATURATED VAPOR PRESSURE Temperature (degrees F) Pounds per square inch N O T P E R T I N E N T It does ignite upon heating in air. It is a dark blue solid.
Slightly soluble in water. Vanadium pentoxide dust is the particulate form of a noncombustible, odorless, yellow-orange or dark gray crystalline solid. Jars, pails, steel drums, fiber drums, bulk bags
Pure karelianite (V2O3) was formed in all samples at saturation of vanadium oxide.
Sodium orthovanadate or sodium vanadium oxide Na. Mis-aisa-The latest News,Tech,Industry,Environment,Low Carbon,Resource,Innovations. Soluble in acids and alkalies Alfa Aesar 11093: Experimental Density: 3. Used as a catalyst. Two solid phases were found, a solid solution of Al 2 O 3 and vanadium oxide and an Al 2 O 3-rich solid phase with 16.7 mass pct V 2 O 3. Vanadium(V) oxide react with sulfuric acid. Further, due to the noted insoluble nature of most phosphate salts, a lower material solubility of SVPO relative to SVO is anticipated. The reaction slowly takes place in a boiling solution. Fire Hazard Excerpt from ERG Guide 151 [Substances - Toxic (Non-Combustible)]: Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Home; About us; Membership & professional community; Campaigning & outreach; Journals, books & databases Slightly soluble in water. large scale application in PSCs. Chemsrc provides vanadium naphthenate oxide(CAS#:68553-60-6) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc. Other Identifiers.
CAS: 1314-62-1 MDL: MFCD00011457 Synonyms: di-Vanadium pentaoxide , Vanadium pentoxide
[ Check the balance ] Vanadium (IV) oxide react with oxygen to produce oxide vanadium (V). Vanadium (IV) oxide acetylacetonate.
Buy Bismuth vanadium oxide (CAS 14059-33-7), a product for proteomics research, from Santa Cruz. FOB Price: US$ 25-50 / kg. Water Solubility: Soluble in acids.
VO.
Explanation of Vanadium(V) oxide.
Highly polycrystalline single-phased solid solutions of V 2-x Bi 2x O 5- were synthesized for mole fractions x varying from 0.01 to 0.04 and at x = 0.05, the compound contorts into an impure phase. Table 1 provides some physicochemical properties of vanadium compounds that are referred to in this review.
MSDS.
Zinc oxide ZnO is used as an additive in many materials and products. vanadic anhydride; sulfate ; divanadium pentoxide Registered trade name(s) Chemical formula V V 2 O 5 VOSO 4 Identification numbers: CAS registry 7440-62-2 1314-62-1 27774-13-6 Solubility: Water Insoluble 1 g dissolves in Soluble in water. Molecular Formula: BiVO4, Molecular Weight: 323.00.
Articles of vanadium naphthenate oxide are included as well. Insoluble in water, soluble in acid and strong alkali. When heated to decomposition, it emits acrid smoke and fumes of vanadium oxides.
It acts as either a metal or non-metal and forms various complex compounds, and it is non-toxic as metal. Compounds containing vanadium in the +3 oxidation state include vanadium oxide (V 2 O 3). Vanadium oxide gels were synthesized via the epoxide-assisted gelation of VOCl. Factory High Purity Vanadium Oxide / Vanadium Pentoxide 1314-62-1 for Catalyst. Dioxovanadium; Vanadium(IV,V) Oxide. The vanadium(IV) oxide is then re-oxidised by the oxygen. Vanadium Trioxide Vanadium trioxide is dark gray crystalline powder, which is of orthorhombic system structure and odorlessness. Vanadium oxide (V 2 O 5) is used as a catalyst in manufacturing sulfuric acid and maleic anhydride and in making ceramics. The solubility of vanadium oxide in the SiO2CaOVOX system was investigated as a function of basicity (CaO/SiO2) at a fixed temperature of 1600C and oxygen partial pressure of 1010 atm. Jinan Boss Chemical Industry Co.,Ltd +86 531 68657995 +86 13280017993; sales@bosschemical.com; HOME; Products. If color is too intense to see the color change, add water.
Examples are V 3 O 7 (VO 2 + V 2 O 5) and V 4 O 7 (2VO 2 + V 2 O 3 ). Thisproduct is recovered in solid form, and is heated to a sufllciently high temperature to eliminate ammonia therefrom and to form substantially pure vanadium oxide. Vanadium pentoxide dust is the particulate form of a noncombustible, odorless, yellow-orange or dark gray crystalline solid. Used to make other chemicals.
Vanadium(IV) oxide 99% trace metals basis; CAS Number: 12036-21-4; EC Number: 234-841-1; Synonyms: Vanadium dioxide; Linear Formula: V2O4; find Sigma-Aldrich-215821 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich
Synonyms.
Vanadium is a mineral.
Thermodynamic properties of substances. Color: Orange To Brown.
Vanadium oxide is a poisonous brown/ yellow solid that is the most stable and abundant compound of vanadium. Vanadium (IV) oxide acetylacetonate MSDS (material safety data sheet) or SDS, CoA and CoQ, dossiers, brochures and other available documents. Vanadium (III) oxide burn fiercely after heating in air. The presence of the reductant gives rise to VOx solution greatly increased this solubility, leading to homoge-neity and sucient consistency of the resulting bers after calcination. Soluble in acids and alkalies Alfa Aesar 11093: Experimental Density: 3. Find here Vanadium Oxide, 1314-62-1 manufacturers & OEM manufacturers India. Name:BISMUTH VANADIUM OXIDE,CAS:14059-33-7.Molecular Fomula:BiO4V,Molar Mass:323.92,Density:6.250,Melting Point:500C,Solubility:Soluble in acids. It appears as a yellow to red crystalline powder. 1.9H 2 O, which may involve structural water or the existence of multiple vanadium oxidation states.
h g 1 at 0.2 A g 1 and exhibits an unprecedented superlong-term cyclic stability with a capacity retention of 96% over 20,000 cycles at 10 A g 1. Vanadium(III) oxide. Insoluble in water, soluble in acid and strong alkali. In Imperial or US customary measurement system, the density is equal to 209.758 pound per cubic foot [lb/ft], or 1.942 ounce per cubic inch [oz/inch] .
Catalog # VA-5630. Jump to main content Jump to site nav.
National Institutes of Health. Periodic table of elements. The solubility of vanadium oxide in the SiO2CaOVOX system was investigated as a function of basicity (CaO/SiO2) at a fixed temperature of Sulfuric acid - concentrated solution.
Vanadium(V) oxide (vanadia) is the chemical compound with the formula V 2 O 5.Commonly known as vanadium pentoxide, this orange solid is the most important [citation needed] compound of vanadium.Upon heating it reversibly loses oxygen. Suitable for Metallurgy and Chemical Industry V2o5 Vanadium Pentoxide.
YW2460000.
NA.
3. Trending.
Anhui Fitech Materials Co., Ltd. View larger video & image. 808505. Skip to content. This reaction takes place at a temperature of 500-600C. Vanadium oxides, a prominent class of the early 3d transition metal compounds, have been objects of great interest in the past few decades due to their very broad range of oxidation states. Thermodynamic properties of substancesThe solubility of the V2O5 decompose to oxygen and vanadium (IV) oxide when 700~1125. Solubility: Soluble In Acid Concentrates. Vanadium oxide is a poisonous brown/ yellow solid that is the most stable and abundant compound of vanadium. VOCl 3 is highly reactive toward water and evolves Cl 2 upon standing.
Storage : Store at room temperature. Though Articles of bismuth vanadium oxide are included as well. Recently, silver vanadium phosphorous oxide (Ag 2 VO 2 PO 4, SVPO) has been studied as possibly combining the desirable thermal stability aspects of LiFePO 4 with the electrical conductivity of SVO.
Notably, the materials from the SVPO family with the lowest vanadium solubility are Ag 2 VO 2 PO 4 and Ag 2 VP 2 O 8. 72720435. Contact Now. Add 50 ml of YELLOW NHVO stock solution to next flask. Vanadium concentrations in the northeastern San Joaquin Valley ranged from <3 g/L to 70 g/L with a median of 21 g/L. Vanadium trioxide undergoes slow air oxidation at room temperature [Kirk-Othmer].
Name: Vanadium oxide (V 2 O 5) IUPAC Systematic Name: Vanadium oxide Synonyms: CI 77938; divanadium pentaoxide; pentaoxodivanadium; vanadic acid anhydride; vanadin (V) oxide (see also Table 1) Solubility: Slightly soluble in water (0.10.8 g/100 cm3); soluble in concentrated The maximum solubility of vanadium oxide was up to 7 mass pct (as V).
Other solubilities: soluble in acids and alkalies,insoluble in alcohol: Formula Weight: 181.88: Percent Purity: 99+% Packaging: Glass bottle: Solubility: Solubility in water: 8g/L. NA. vanadium processing, preparation of the metal for use in various products.
Vanadium (V) oxide is amphoteric oxide, but mainly acid. The solubility controls on vanadium (V) in groundwater were studied due to concerns over possible harmful health effects of ingesting V in drinking water. This represents the first epoxide-assisted gelation developed for vanadium oxide gels and serves as a potential economical replacement for the fabrication of vanadium oxide gels via the hydrolysis of vanadium alkoxides.
In oxic groundwater, speciation modeling