Bohr Model & Atomic Spectra Overview & Examples | What is Bohr's Model? What is atomic number, mass number and atomic mass? Mass Defect Formula & Examples | What is Nuclear Mass Defect?
Which of the following elements of matter would best class 9 chemistry CBSE, Which one of these does not sublime on heating A Ammonium class 9 chemistry CBSE, How do solids liquids and gases differ in shape and class 9 chemistry CBSE, Which of the following does not belong to the group class 9 chemistry CBSE, Fire can be controlled by removing which of the following class 9 chemistry CBSE, Why does water vapour play a role in heating and cooling class 9 chemistry CBSE, What was the capital of Kanishka A Mathura B Purushapura class 7 social studies CBSE, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers. 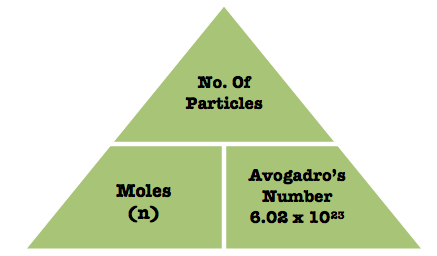 Understand how to balance chemical equations step by step with examples. Also, learn how light behaves as waves as well as particles. 53Mn is radioactive with a half-life of 3.7(2) Ma, too short for survival of a detectable amount CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. How to Convert Units in the Metric System. Learn about covalent bonds. Astronomy is the scientific study of celestial bodies in outer space. Learn how to define acids and bases, explore the pH scale, and discover how to find pH values. the standard atomic weight.
Understand how to balance chemical equations step by step with examples. Also, learn how light behaves as waves as well as particles. 53Mn is radioactive with a half-life of 3.7(2) Ma, too short for survival of a detectable amount CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. How to Convert Units in the Metric System. Learn about covalent bonds. Astronomy is the scientific study of celestial bodies in outer space. Learn how to define acids and bases, explore the pH scale, and discover how to find pH values. the standard atomic weight.  Calculate the mass number of an atom that is Ionic bonds are electrostatic interactions between two oppositely charged ions. This number will change with each isotope of an element as the number of neutrons will change but the number of protons remains the same. Physical vs. Chemical Changes in Matter | Overview, Comparisons & Differences. Ar(Mn) = 54.938 043(2) since 2017 What is a chemical bond? Consider the atom designated by {207}{80} A Se atom has a mass number of 75. Become a Study.com member to unlock this answer! Learn what light is and how it is divided into various visible and invisible spectrums. Manganese is a monoisotopic element and its atomic weight is determined solely by its isotope 55Mn. See the concept mass number vs atomic mass. Elements in Science: Overview & Examples | What are Elements? Createyouraccount. Tropic of Cancer passes through how many states? Learn about chemical bonding and how ionic bonds form, discover the properties of electronegativity, and review examples of elements containing ionic bonds. It was discovered by the Swedish pharmacist and chemist Carl-Wilhelm Scheele in 1774. Learn about Niels Bohr's atomic model and compare it to Rutherford's model. Atom Overview, Structure & Examples | What is an Atom? In the same year, Discover how to find atomic mass, and learn what an atomic number represents. Compounds and molecules are built from elements composed of at least two atoms joined with a chemical bond.
Calculate the mass number of an atom that is Ionic bonds are electrostatic interactions between two oppositely charged ions. This number will change with each isotope of an element as the number of neutrons will change but the number of protons remains the same. Physical vs. Chemical Changes in Matter | Overview, Comparisons & Differences. Ar(Mn) = 54.938 043(2) since 2017 What is a chemical bond? Consider the atom designated by {207}{80} A Se atom has a mass number of 75. Become a Study.com member to unlock this answer! Learn what light is and how it is divided into various visible and invisible spectrums. Manganese is a monoisotopic element and its atomic weight is determined solely by its isotope 55Mn. See the concept mass number vs atomic mass. Elements in Science: Overview & Examples | What are Elements? Createyouraccount. Tropic of Cancer passes through how many states? Learn about chemical bonding and how ionic bonds form, discover the properties of electronegativity, and review examples of elements containing ionic bonds. It was discovered by the Swedish pharmacist and chemist Carl-Wilhelm Scheele in 1774. Learn about Niels Bohr's atomic model and compare it to Rutherford's model. Atom Overview, Structure & Examples | What is an Atom? In the same year, Discover how to find atomic mass, and learn what an atomic number represents. Compounds and molecules are built from elements composed of at least two atoms joined with a chemical bond. Understand what an electron shell is by learning the electron shell definition. Name them.
 Learn how fast light travels and find the speed of light in meters or miles per second. All other trademarks and copyrights are the property of their respective owners. Mass number is a count of the total number of protons and neutrons found in the nucleus of an atom. Understand what nuclear mass defect in chemistry is, its function, and its importance. Balancing chemical equations is possible because of the law of conservation of matter. Understand the definition of atomic mass, atomic number, and atomic weight. Balancing Chemical Equations | Overview, Chemical Reactions & Steps. Explore atoms. Ionic Bond Formation, Types & Examples | What is an Ionic Bond? This differs from the atomic mass located on the periodic table of elements which is actually the average of the mass numbers of all atoms of that element in nature. Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE. See atom examples and find the elements they form. High School Chemistry: Homework Help Resource, College Chemistry: Homework Help Resource, ILTS Science - Chemistry (106): Test Practice and Study Guide, High School Physical Science: Homework Help Resource, High School Physical Science: Tutoring Solution, NY Regents Exam - Chemistry: Help and Review, Glencoe Chemistry - Matter And Change: Online Textbook Help, Intro to Physics for Teachers: Professional Development, All Teacher Certification Test Prep Courses, Working Scholars Bringing Tuition-Free College to the Community. Atomic weights of the elements: Review 2000. How Compounds and Molecules Are Built From Elements. Explore their types and discover examples of covalent molecules. What are the two main types of chemical bonds? What element's atomic number is 45? Manganese Learn about the definition and history of astronomy, and discover the different fields of astronomy -- planetary, stellar, solar, observational, and theoretical. Learn what the properties of gases are and discover the kinetic molecular theory of gases. All rights reserved. Explore how to draw the Bohr model of hydrogen and argon, given their electron shells. Understand the definition of covalent bonds and how they are formed. Learn what atomic number, mass number and atomic mass represent. Learn how ionic bonds are formed and what holds ionic compounds together. Electron Shell Overview & Energy Levels | What is an Electron Shell? Name the Largest and the Smallest Cell in the Human Body ? Learn about chemical bonding, explore how hydrogen bonds form, discover the differences between intramolecular forces and intermolecular forces, then review an example of how these bonds are used. Understand the significance of light-years and lookback time with examples. copyright 2003-2022 Study.com. Acids are substances that contribute molecules, while bases are substances that can accept them. When using the metric system, it is helpful to know how to convert units from other systems. In this lesson, explore the metric system, how to set up a conversion, and how to calculate those units, with some additional practice. Know its formula and learn how to compute it through given examples. Learn how elemental personalities build compounds and molecules, explore ionic and covalent chemical bonds, and compare pure substances to mixtures. Learn basic information about what an element is, what they are made of, how and why they are different, and how scientists have organized them into the periodic table. The name derives from the Latin magnes for "magnet" since pyrolusite (MnO2) has magnetic properties. Understand the characteristics of ideal gases and know examples of the properties of gases. Learn about ionic vs covalent bonds, chemical bond examples, and the difference between ionic and covalent bonds. Covalent Bonds Formation & Examples | What is a Covalent Bond? Get access to this video and our entire Q&A library. The mass number of manganese is 55. Hydrogen bonds are electrostatic interactions that occur when a hydrogen atom binds to an electronegative atom. the Swedish chemist Johan Gottlieb Gahn first isolated the metal. Isotopes & Atomic Mass: Overview & Examples | What is Atomic Mass? The Commission last revised the standard atomic weight of manganese in 2017 based on the latest Atomic Mass Evaluation by IUPAP. In Our experts can answer your tough homework and study questions. If no isotope is specified, using the name of the element refers to the most abundant isotope of that element. What is the difference between an ionic and a covalent bond? one disintegration per minute per gram of manganese in sediment cores corresponding to an abundance of 3x10-13, much too small to affect of primordial isotope. Learn the definition of an atom and understand its structure with diagrams and explanations. Study physical change vs. chemical change examples and processes. However, 53Mn has been identified on earth as a cosmic-ray product and as a constituent Types of Chemical Bonds: Ionic vs Covalent | Examples of Chemical Bonds. Learn about the electron shell energy levels and the valence electrons. of cosmic dust amounting to approx. Kinetic Molecular Theory of Gases | Properties, Characteristics & Examples. Speed of Light: Distance & Light-Years | What is the Speed of Light? Learn about changes in matter and see how physical changes of matter compare to chemical changes.
Learn how fast light travels and find the speed of light in meters or miles per second. All other trademarks and copyrights are the property of their respective owners. Mass number is a count of the total number of protons and neutrons found in the nucleus of an atom. Understand what nuclear mass defect in chemistry is, its function, and its importance. Balancing chemical equations is possible because of the law of conservation of matter. Understand the definition of atomic mass, atomic number, and atomic weight. Balancing Chemical Equations | Overview, Chemical Reactions & Steps. Explore atoms. Ionic Bond Formation, Types & Examples | What is an Ionic Bond? This differs from the atomic mass located on the periodic table of elements which is actually the average of the mass numbers of all atoms of that element in nature. Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE. See atom examples and find the elements they form. High School Chemistry: Homework Help Resource, College Chemistry: Homework Help Resource, ILTS Science - Chemistry (106): Test Practice and Study Guide, High School Physical Science: Homework Help Resource, High School Physical Science: Tutoring Solution, NY Regents Exam - Chemistry: Help and Review, Glencoe Chemistry - Matter And Change: Online Textbook Help, Intro to Physics for Teachers: Professional Development, All Teacher Certification Test Prep Courses, Working Scholars Bringing Tuition-Free College to the Community. Atomic weights of the elements: Review 2000. How Compounds and Molecules Are Built From Elements. Explore their types and discover examples of covalent molecules. What are the two main types of chemical bonds? What element's atomic number is 45? Manganese Learn about the definition and history of astronomy, and discover the different fields of astronomy -- planetary, stellar, solar, observational, and theoretical. Learn what the properties of gases are and discover the kinetic molecular theory of gases. All rights reserved. Explore how to draw the Bohr model of hydrogen and argon, given their electron shells. Understand the definition of covalent bonds and how they are formed. Learn what atomic number, mass number and atomic mass represent. Learn how ionic bonds are formed and what holds ionic compounds together. Electron Shell Overview & Energy Levels | What is an Electron Shell? Name the Largest and the Smallest Cell in the Human Body ? Learn about chemical bonding, explore how hydrogen bonds form, discover the differences between intramolecular forces and intermolecular forces, then review an example of how these bonds are used. Understand the significance of light-years and lookback time with examples. copyright 2003-2022 Study.com. Acids are substances that contribute molecules, while bases are substances that can accept them. When using the metric system, it is helpful to know how to convert units from other systems. In this lesson, explore the metric system, how to set up a conversion, and how to calculate those units, with some additional practice. Know its formula and learn how to compute it through given examples. Learn how elemental personalities build compounds and molecules, explore ionic and covalent chemical bonds, and compare pure substances to mixtures. Learn basic information about what an element is, what they are made of, how and why they are different, and how scientists have organized them into the periodic table. The name derives from the Latin magnes for "magnet" since pyrolusite (MnO2) has magnetic properties. Understand the characteristics of ideal gases and know examples of the properties of gases. Learn about ionic vs covalent bonds, chemical bond examples, and the difference between ionic and covalent bonds. Covalent Bonds Formation & Examples | What is a Covalent Bond? Get access to this video and our entire Q&A library. The mass number of manganese is 55. Hydrogen bonds are electrostatic interactions that occur when a hydrogen atom binds to an electronegative atom. the Swedish chemist Johan Gottlieb Gahn first isolated the metal. Isotopes & Atomic Mass: Overview & Examples | What is Atomic Mass? The Commission last revised the standard atomic weight of manganese in 2017 based on the latest Atomic Mass Evaluation by IUPAP. In Our experts can answer your tough homework and study questions. If no isotope is specified, using the name of the element refers to the most abundant isotope of that element. What is the difference between an ionic and a covalent bond? one disintegration per minute per gram of manganese in sediment cores corresponding to an abundance of 3x10-13, much too small to affect of primordial isotope. Learn the definition of an atom and understand its structure with diagrams and explanations. Study physical change vs. chemical change examples and processes. However, 53Mn has been identified on earth as a cosmic-ray product and as a constituent Types of Chemical Bonds: Ionic vs Covalent | Examples of Chemical Bonds. Learn about the electron shell energy levels and the valence electrons. of cosmic dust amounting to approx. Kinetic Molecular Theory of Gases | Properties, Characteristics & Examples. Speed of Light: Distance & Light-Years | What is the Speed of Light? Learn about changes in matter and see how physical changes of matter compare to chemical changes.