Br J Dermatol. J Invest Dermatol. In the case of the immunoproteasome, activity of the 26s proteasome is decreased, leading to lack of ubiquinated protein degradation and aggregation of the proteasome, polyubiquinated proteins and associated heat shock proteins.390 These three mechanisms combine to cause formation of the MDB intracellular aggregates. The 10-nm filaments of the inner root sheath cells fail to react with any of our monoclonal antibodies and are therefore immunologically distinguishable from the cortex and cuticle filaments. Classical type II epithelial keratins were assigned the numbers K1K8. Our AE14 antibody recognizes a group of 10-25K hair proteins which most likely corresponds to the high sulfur proteins, our AE12 and AE13 antibodies define a doublet of 44K/46K proteins which are relatively acidic and correspond to the type I low sulfur keratins, and our previously described AE3 antibody recognizes a triplet of 56K/59K/60K proteins which are relatively basic and correspond to the type II low sulfur keratins.
Another study on keratinocytes lacking keratin networks showed that the cell body Young's modulus of these cells is lowered by 40% as measured with AFM, and their intracytoplasmic viscosity is 40% lower as assessed with magnetic tweezers (Ramms et al., 2013). Follicle group 1B proteins are postulated to have arisen from the trichohyalin droplets of the developing medulla and inner-root-sheath layers of the follicle and may be precursors of the proteins of the mature medullaand inner root sheath.
This product is furnished for LABORATORY RESEARCH USE ONLY.Not for diagnostic or therapeutic use. MDBs exhibit aberrant cross-linking, increased phosphorylation, partial proteolytic degradation, and an increase of -sheet conformation. Although numerous hair proteins have been studied biochemically and many have been sequenced, relatively little is known about their in situ distribution and differential expression in the hair follicle. 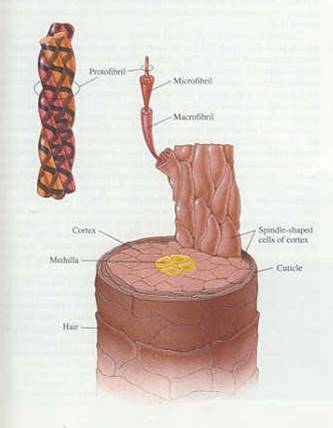 government site. Accessibility HOXC13 is another transcription factor that is implicated in the regulation of hair keratins. High quality antibodies with extensive validation data. The range of keratins that has evolved gives rise to unique pairing of types I and II keratins which are signature to the specific tissue and its state of differentiation. anticorps-enligne.fr, chinese () Expected to show broad specificity. The main difference between hair and epithelial keratins is that hair keratins possess a greater amount of sulfur in their head and tail domains, which enables them to form tight cross-linking with keratin-associated proteins (KAPs) (14) that contribute to the hard structure of both hair and nails. Our scientific customer service is always at your disposal if you have any questions about the selection or application of our products. Store the pan Cytokeratin antibody at 2-8C (with azide) or aliquot and store at -20C or colder (without azide). The https:// ensures that you are connecting to the
government site. Accessibility HOXC13 is another transcription factor that is implicated in the regulation of hair keratins. High quality antibodies with extensive validation data. The range of keratins that has evolved gives rise to unique pairing of types I and II keratins which are signature to the specific tissue and its state of differentiation. anticorps-enligne.fr, chinese () Expected to show broad specificity. The main difference between hair and epithelial keratins is that hair keratins possess a greater amount of sulfur in their head and tail domains, which enables them to form tight cross-linking with keratin-associated proteins (KAPs) (14) that contribute to the hard structure of both hair and nails. Our scientific customer service is always at your disposal if you have any questions about the selection or application of our products. Store the pan Cytokeratin antibody at 2-8C (with azide) or aliquot and store at -20C or colder (without azide). The https:// ensures that you are connecting to the  squamous vs. adenocarcinoma of the lung, liver carcinoma, breast cancer, and esophageal cancer. Etats-Unis, Tel +1 877 302 8632 Mark M. Tran, Bernard A. Cohen, in Avery's Diseases of the Newborn (Ninth Edition), 2012. Tel: 770-729-2992, 1-888-494-8555 (toll-free). The production of the different keratins is a tightly regulated process that involves several transcription factors and proteins. Specific Keratins and their Associated Proteins as Markers for Hair Follicle Differentiation. Thirty positions, 4170 were proposed to be sufficient to cover all new type I keratins that may be discovered in the future. Keratins of the human hair follicle: "hyperproliferative" keratins consistently expressed in outer root sheath cells in vivo and in vitro. It may be useful to characterize the source of various neoplasms and to study the distribution of cytokeratin containing cells in epithelia during normal development and during the development of epithelial neoplasms. Keratin assembly proceeds strictly from type Itype II heterodimers, accounting for the duality of sequences as well as their pairwise regulation in vivo. In mammals, specific type Itype II gene pairings occur in simple (e.g., liver and GI tract), complex (e.g., skin and oral mucosa), and hard epithelia (e.g., hair and nail), with little overlap. Hum Mol Genet. Would you like email updates of new search results? 2018 Nov 21;9(1):4903. doi: 10.1038/s41467-018-07142-9. doi: 10.1111/1523-1747.ep12540831. Yu DW, Pang SY, Checkla DM, Freedberg IM, Sun TT, Bertolino AP. Our AE14 antibody recognizes a group of 10-25K hair proteins which most likely corresponds to the high sulfur proteins, our AE12 and AE13 antibodies define a. Expression of hair keratins in the adult nail unit: an immunohistochemical analysis of the onychogenesis in the proximal nail fold, matrix and nail bed. Genomic landscape of a metastatic malignant proliferating tricholemmal tumor and its response to PI3K inhibition. The human genome features 54 functional keratin genes, with 28 type I and 26 type II keratin genes (see Table 1). This finding is consistent with studies of vimentin listed above that report a larger effect of disrupting IFs when cells are indented closer to the perinuclear region. HHS Vulnerability Disclosure, Help Accordingly, the basic-to-neutral type II keratins were grouped as K1K8 and the acidic, During normal assembly of a keratin intermediate filament (KIF), a, https://www.mechanobio.info/figure/1384241639768/, Type I and type II IFs are part of the keratin (or cytokeratin) family of proteins found in all epithelia. The numbering of additional type II epithelial keratins should begin with K71. 2019 Apr;28(4):332-344. doi: 10.1111/exd.13913. Keratin assembly proceeds strictly from type I to type II heterodimers, accounting for the duality of sequences as well as their pairwise regulation in vivo. Mazen Kurban, Angela M. Christiano, in Emery and Rimoin's Principles and Practice of Medical Genetics (Sixth Edition), 2013. In a variety of disease conditions, alterations in hepatocellular keratin assembly occur. Differentiation; research in biological diversity.
squamous vs. adenocarcinoma of the lung, liver carcinoma, breast cancer, and esophageal cancer. Etats-Unis, Tel +1 877 302 8632 Mark M. Tran, Bernard A. Cohen, in Avery's Diseases of the Newborn (Ninth Edition), 2012. Tel: 770-729-2992, 1-888-494-8555 (toll-free). The production of the different keratins is a tightly regulated process that involves several transcription factors and proteins. Specific Keratins and their Associated Proteins as Markers for Hair Follicle Differentiation. Thirty positions, 4170 were proposed to be sufficient to cover all new type I keratins that may be discovered in the future. Keratins of the human hair follicle: "hyperproliferative" keratins consistently expressed in outer root sheath cells in vivo and in vitro. It may be useful to characterize the source of various neoplasms and to study the distribution of cytokeratin containing cells in epithelia during normal development and during the development of epithelial neoplasms. Keratin assembly proceeds strictly from type Itype II heterodimers, accounting for the duality of sequences as well as their pairwise regulation in vivo. In mammals, specific type Itype II gene pairings occur in simple (e.g., liver and GI tract), complex (e.g., skin and oral mucosa), and hard epithelia (e.g., hair and nail), with little overlap. Hum Mol Genet. Would you like email updates of new search results? 2018 Nov 21;9(1):4903. doi: 10.1038/s41467-018-07142-9. doi: 10.1111/1523-1747.ep12540831. Yu DW, Pang SY, Checkla DM, Freedberg IM, Sun TT, Bertolino AP. Our AE14 antibody recognizes a group of 10-25K hair proteins which most likely corresponds to the high sulfur proteins, our AE12 and AE13 antibodies define a. Expression of hair keratins in the adult nail unit: an immunohistochemical analysis of the onychogenesis in the proximal nail fold, matrix and nail bed. Genomic landscape of a metastatic malignant proliferating tricholemmal tumor and its response to PI3K inhibition. The human genome features 54 functional keratin genes, with 28 type I and 26 type II keratin genes (see Table 1). This finding is consistent with studies of vimentin listed above that report a larger effect of disrupting IFs when cells are indented closer to the perinuclear region. HHS Vulnerability Disclosure, Help Accordingly, the basic-to-neutral type II keratins were grouped as K1K8 and the acidic, During normal assembly of a keratin intermediate filament (KIF), a, https://www.mechanobio.info/figure/1384241639768/, Type I and type II IFs are part of the keratin (or cytokeratin) family of proteins found in all epithelia. The numbering of additional type II epithelial keratins should begin with K71. 2019 Apr;28(4):332-344. doi: 10.1111/exd.13913. Keratin assembly proceeds strictly from type I to type II heterodimers, accounting for the duality of sequences as well as their pairwise regulation in vivo. Mazen Kurban, Angela M. Christiano, in Emery and Rimoin's Principles and Practice of Medical Genetics (Sixth Edition), 2013. In a variety of disease conditions, alterations in hepatocellular keratin assembly occur. Differentiation; research in biological diversity.
Non-polar protofilaments subsequently result from the elongation of tetramers. 1). Keratin 17 expression in the hard epithelial context of the hair and nail, and its relevance for the pachyonychia congenita phenotype. The human and mouse genomes have >50 functional keratin genes, with a slightly larger number of type I keratin genes (see Table I). EHK is transmitted in an autosomal dominant fashion, with a high rate of spontaneous mutation. Using these and other immunological probes, we demonstrate the following. The conservation of keratins in terms of primary structure and distribution suggests an important role in epithelial diversity this role has yet to be defined and does not lie in the execution of differentiation. Reflecting a partial or complete loss of this crucial scaffolding role, genetic mutations altering the coding sequence of keratins account for a large number of epithelial fragility disorders. The central rod domain of keratins is the main determinant of polymerization, with additional contributions provided by the head domain. The central rod domain of keratins is the main determinant of polymerization, with an additional contribution provided by the head domain, whereas the tail domain is largely dispensable for this purpose.
It is a broad spectrum anti pan-cytokeratin antibody, which differentiates epithelial tumors from non-epithelial tumors e.g. Human and bovine hair follicles. genomics-online.com. Their molecular weight ranges from 40 kDa (K19) to 64 kDa (K9). The protein encoded by this gene is a member of the keratin gene family. They form intracellular intermediate filament networks which provide a scaffold for epithelial cells and tissues to withstand mechanical stress and maintain structural integrity.26b,27b,32,33 They span across the cytoplasm and tend to be denser in the peri-nuclear regions to ensure mechanical resilience, protect against variations in hydrostatic pressure and also help to establish cell polarity. By mass spectrometry, MDBs consist of keratin, ubiquitinated keratin, the stress-induced and ubiquitin-binding protein p62, heat shock proteins (HSPs) 70 and 25, and other peptides.388 Three mechanisms appear to be involved in formation of MDB:389 epigenetic changes in gene expression following exposure of hepatocytes to a toxic environment; a shift from the 26s proteasome to an immunoproteasome; and chronic activation of the toll-like signalling pathways which stimulate proinflammatory and cell growth pathways. Misfolded and aggregated keratins can accumulate, clumped together in a characteristic intracellular inclusion, MalloryDenk bodies (MDBs, Fig. Numbers K81K86 were assigned to the six type II human hair keratins. This site needs JavaScript to work properly. Kelsie M. Bernot, Pierre A. Coulombe, in Encyclopedia of Biological Chemistry, 2004. Molecular analysis of affected families has identified genetic mutations in the genes encoding the synthesis of the keratin filaments that are preferentially expressed in the superficial epidermis, K1 (located within the type I keratin gene cluster at chromosome 17q) and K10 (located within the type II keratin gene cluster at chromosome 12q) (Francis, 1994; Nirunsuksiri et al, 1995; Smack et al, 1994; Steijlen et al, 1994a). Intermediate filaments are built from monomers that associate with each other form dimers. Disclaimer, National Library of Medicine The nomenclature also allows for the inclusion of a fourth category of non-human epithelial and hair keratins of other mammalian species, whose genes are either absent or occur as pseudogenes in the human genome. Pierre A. Coulombe, Kelsie M. Bernot, in Encyclopedia of Biological Chemistry, 2004. The extraordinary stability of heterodimers (some of them resist 10 M urea!) 2004 Aug;151(2):362-71. doi: 10.1111/j.1365-2133.2004.06108.x. | KRT5 | KRT6A | KRT6D | KRT8 | KRTA | KRTB, SIMOA - Single Molecule Protein Detection, Comprehensive Service and Discovery Center, Bulk Monoclonal Antibody Production Service. This antibody stains cytokeratins present in normal and abnormal human tissues and has high sensitivity in the recognition of epithelial cells and carcinomas. The present data indicate that keratins can also be expressed by other stratified epithelia during vitamin A deficiency- induced keratinization, and suggest the possibility that they may play a role in the formation of the densely packed tonofilament bundles in cornified cells of keratinized tissues. Rabbit anti-Human Pan Cytokeratin Antibody/Acidic + Basic. Annals of the New York Academy of Sciences. Limerick, PA 19464 In mammals, specific type Itype II gene pairings occur in simple (e.g., liver, GI tract), complex (e.g., skin, oral mucosa) and hard epithelia (e.g., hair, nail), with little overlap. -. Fax +1 888 205 9894 (Toll-free), german (deutsch)
V. Singh, K.W. Recent Pat Drug Deliv Formul. The disorder also can be expressed in mosaic fashion as an epidermal nevus oriented along the lines of Blaschko (Paller, 1994). By continuing you agree to the use of cookies. FOIA From: Encyclopedia of Biological Chemistry, 2004, Elisabeth E. Charrier, Paul A. Janmey, in Methods in Enzymology, 2016. Not surprisingly, most of the intracellular pool of keratin proteins (>95%) is in the polymerized form. Variation and frequency of cytokeratin polypeptide patterns in human squamous non-keratinizing epithelium. Accordingly, the basic-to-neutral type II keratins were grouped as K1K8 and the acidic type I keratins as K9K19. https://en.wikipedia.org/w/index.php?title=Type_I_keratin&oldid=929810284, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 8 December 2019, at 10:55. Type I keratins tend to be smaller (4064 kDa) and acidic (pI4.76.1) compared to type II, which are larger (5268kDa) and basic-neutral (pI5.48.4) in charge. Kelsie M. Bernot, Hani Zaher, in Encyclopedia of Biological Chemistry (Third Edition), 2021. Ng, in Comprehensive Biomaterials II, 2017, In humans, keratins are encoded by 54 distinct functional genes out of the total of 70 human intermediate filament genes thereby making it the largest sub family (Types I and II out of VI in the human intermediate filament family). A key degradation pathway for misfolded proteins is the ubiquitinationproteasome pathway. We use cookies to help provide and enhance our service and tailor content and ads. Monoclonal Antibody Studies of Mammalian Epithelial Keratins: A Review a, Correlation of specific keratins with different types of epithelial differentiation: Monoclonal antibody studies, Although numerous hair proteins have been studied biochemically and many have been sequenced, relatively little is known about their in situ distribution and differential expression in the hair follicle. Transient expression of mouse hair keratins in transfected HeLa cells: interactions between "hard" and "soft" keratins. 1985 Mar;52(3):243-56 Modulation of keratin expression also depends upon both the type and stage of differentiation in epithelia. official website and that any information you provide is encrypted 3848 | 3850 | 3851 | 3852 | 3853 | 3856 | 3858 | 3861 | 3866 | 3868, CYK8 | K6A | KPP | KRT1 | KRT10 | KRT14 | KRT15 | KRT16 | KRT16A | KRT3, Keratin, type II cytoskeletal 1 (67 kDa cytokeratin) (Cytokeratin-1) (CK-1) (Hair alpha protein) (Keratin-1) (K1) (Type-II keratin Kb1),Keratin, type II cytoskeletal 3 (65 kDa cytokeratin) (Cytokeratin-3) (CK-3) (Keratin-3) (K3) (Type-II keratin Kb3),Keratin, type II cytoskeletal 3 (65 kDa cytokeratin) (Cytokeratin-3) (CK-3) (Keratin-3) (K3) (Type-II keratin Kb3),Keratin, type II cytoskeletal 5 (58 kDa cytokeratin) (Cytokeratin-5) (CK-5) (Keratin-5) (K5) (Type-II keratin Kb5),Keratin, type II cytoskeletal 6A (Cytokeratin-6A) (CK-6A) (Cytokeratin-6D) (CK-6D) (Keratin-6A) (K6A) (Type-II keratin Kb6) (allergen Hom s 5),Keratin, type II cytoskeletal 8 (Cytokeratin-8) (CK-8) (Keratin-8) (K8) (Type-II keratin Kb8),Keratin, type I cytoskeletal 10 (Cytokeratin-10) (CK-10) (Keratin-10) (K10),Keratin, type I cytoskeletal 14 (Cytokeratin-14) (CK-14) (Keratin-14) (K14),Keratin, type I cytoskeletal 15 (Cytokeratin-15) (CK-15) (Keratin-15) (K15),Keratin, type I cytoskeletal 16 (Cytokeratin-16) (CK-16) (Keratin-16) (K16), 0.2 mg/ml in 1X PBS with 0.1 mg/ml BSA (US sourced) and 0.05% sodium azide. Search, find and order tools with extensive validation data, images, references. Mingorance lvarez E, Martnez Quintana R, Prez Pico AM, Mayordomo R. Biology (Basel).
A recent study has shown that these genetic mutations were due to a splice site and deletion mutations of the types I and 10 of keratin (Virtanen et al, 2003). Double optical trapping experiments performed on suspended murine keratinocytes without keratin filaments show that deformability of these cells is increased about 60% (Seltmann et al., 2013). Gallant JN, Sewell A, Almodovar K, Wang Q, Dahlman KB, Abramson RG, Kapp ME, Brown BT, Boyd KL, Gilbert J, Cohen DN, Yarbrough WG, Zhao Z, Lovly CM. 1984;28(1):30-5 SPC treatment reduced the elastic moduli of treated cells, characterized with a parallel microplate cell stretcher, by 40%, and enhanced these cells ability to squeeze through small pores in a size-limited migration assay. antibodies-online Inc. Type I keratins are encoded on chromosome 17q and encompasses: K9, K10, K11, K12, K13, K14, K15, K16, K17, K18, K19 and K20. Keratins are extraordinarily resistant to digestion by the usual proteolytic enzymes and are insoluble in water, diluted acid solutions, alkalis, and other organic solvents. While any combination of purified type I and type II keratin proteins yields a fibrous polymer in vitro, specific pairs are coregulated in a fashion directly related to epithelial tissue type and/or differentiation state in vivo. However, they can be solubilized in the presence of denaturing agents such as urea.41. Characterization of the proteins of guinea-pig hair and hair-follicle tissue. Expression of specific keratin markers by rabbit corneal, conjunctival, and esophageal epithelia during vitamin A deficiency. 1.32). The site is secure.
In fact, the availability of a wide range of keratin subtypes with the possibility of a huge permutation of combinations provides nature with a versatile toolset to create the vast diversity of epithelia tissues we know today. The most commonly affected keratins are keratin 33 and keratins 81, 85, and 86 (1). Several additional roles for keratin proteins, affecting cell growth, migration, programmed cell death, and response to stresses, are manifested in a sequence- and context-dependent fashion and play key roles in normal physiology and in disease (Fuchs, 1995). These heterodimers then interact along their lateral surfaces and then in an end-to-end fashion to give rise to the 10-nm-wide filaments. sharing sensitive information, make sure youre on a federal Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. Recent Patents on Permeation Enhancers for Drug Delivery Through Nails. -, Differentiation. Please enable it to take advantage of the complete set of features! -, Lab Invest. 1991 Aug;97(2):354-63. doi: 10.1111/1523-1747.ep12480706. Thus the numbers 928 represent the 17 type I human epithelial keratins. Ballooning of hepatocytes is accompanied by a reduced density or even loss of the cytoplasmic IF network. The molecular biology of intermediate filaments. An updated classification of hair follicle morphogenesis. The four recently identified type I epithelial keratins were assigned the numbers 2528 after the classical type I epithelial keratins (K9K24). This protein-related article is a stub. Bethesda, MD 20894, Web Policies -, Proc Natl Acad Sci U S A. To study this problem, we have prepared several mouse monoclonal antibodies that recognize different classes of human hair proteins. Copyright 2022 Elsevier B.V. or its licensors or contributors. Type I keratins (or Type I cytokeratins) are cytokeratins that constitute the Type I intermediate filaments (IFs) of the intracytoplasmatic cytoskeleton, which is present in all mammalian epithelial cells. Most of the type I keratins consist of acidic, low molecular weight proteins which in vivo are arranged in pairs of heterotypic Type I and Type II keratin chains, coexpressed during differentiation of simple and stratified epithelial tissues. 1984;37(1-2):17-23 1. FOXN1 plays a crucial role in the activation of genes involved in cortex and IRS differentiation.
Type I keratins tend to be smaller and acidic compared to the larger, neutralbasic type II keratins.