{ HW: The Mole and Avogadros Number (back of WS) Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. "@type": "ImageObject", ", "@context": "http://schema.org", "name": "The Mole Triangle Mole \uf0e0 Gram Conversions", "width": "1024" What is the mass of \(7.50 \: \text{mol}\) of (calcium oxide) \(\ce{CaO}\)? 1 Chapter 10 Chemical Quantities 10.2 The Mole Copyright 2008 by Pearson Education, Inc. Publishing as Benjamin Cummings. How many pencils are in a gross? ", 3\/12\/14. Write down the formula for the substance, and calculate the mass of that ONE MOLE. To calculate the mass of a higher number of moles, or even calculate the number of moles in a certain mass, a formula triangle can be used. ", The counting unit of ChemistryMole The counting unit of Chemistry (i.e. What does a monkey call barium with two sodium atoms BaNaNa. How many moles of Al2O3 will be produced when 23.9 g of H2O are reacted according to this chemical equation? The balanced chemical equation is as follows. }, 14 "name": "Exit Ticket How many bananas are in a mole of bananas", The following example illustrates both techniques.
The simplest type of manipulation using molar mass as a conversion factor is a mole-mass conversion (or its reverse, a mass-mole conversion). }, 12 mol \uf0e0 L and back. "@context": "http://schema.org", Ammonium nitrate decomposes to dinitrogen monoxide and water according to the following equation. "@context": "http://schema.org", \uf04a", BaNaNa Drill Leave your HW on the desk Take out a sheet of notebook paper. Drill. "@context": "http://schema.org", Thenand only thenwe use the balanced chemical equation to construct a conversion factor to convert that quantity to moles of another substance, which in turn can be converted to a corresponding mass. }, 5 As usual, we start with the quantity we were given: \[\mathrm{3.59\: \cancel{ mol\: Fe_2O_3 } \times \left( \dfrac{3\: mol\: SO_3}{1\: \cancel{ mol\: Fe_2O_3}} \right) =10.77\: mol\: SO_3} \label{Eq2}\]. Unit: Chemical Quantities Counting Representative Particles with the Mole Day 1 - Notes. Our tips from experts and exam survivors will help you through. "contentUrl": "https://slideplayer.com/slide/12860497/78/images/6/Item+Number+Mass+%28g%29.jpg", To produce 27.6 mol of H2O, 13.8 mol of O2 react. Each time you give feedback, TPT gives you feedback credits that you use to lower the cost of your future purchases. "contentUrl": "https://slideplayer.com/slide/12860497/78/images/11/Calculate+number+of+atoms+in+2.5+mol+Kr..jpg", "description": "If I gave you one dollar a second, how long do you think it would take for me to give you a mole of dollars (i.e. 2022 SlidePlayer.com Inc. All rights reserved. "@type": "ImageObject",
{ "name": "Would you like a mole of $", Read about our approach to external linking. TextMap: The Basics of GOB Chemistry (Ball et al. 1 mole eggs = x 1023 eggs! \(\cancel{4.20 \: \text{mol} \: H_2} \times \dfrac{2 \: \text{mol} \: NH_3}{\cancel{3 \: \text{mol} \: H_2}} = 2.80 \: \text{mol} \: NH_3\), The reaction of \(4.20 \: \text{mol}\) of hydrogen with excess nitrogen produces \(2.80 \: \text{mol}\) of ammonia. Different units of measurement for this formula triangle is: Formula triangle to find out mass, moles and formula mass, Formula triangle to find out no. Use the mole triangle to convert from moles to grams, moles to particles, or moles to liters. How to get TPT credit to use on future purchases, Please go to your My Purchases page (you may need to login).
)",
By the same token, the ratios we constructed to describe molecules reaction can also be constructed in terms of moles rather than molecules. You will go to the lab area\u2014there are graduated cylinders that each contain ONE MOLE of a substance.
\end{array}\]. For this unit of quantitative chemistry, there are 3 key formulas for mole conversion that you need to remember. You know that one dozen of any item is ______. "name": "The counting unit of Chemistry", "description": "Convert 32 g O2 to grams. "@type": "ImageObject", Write down the formula for the substance, and calculate the mass of that ONE MOLE. $6.022 x 1023) It would take over 19,100,000,000,000,000 years.
"@context": "http://schema.org", "@context": "http://schema.org", You will now receive email updates about this store. The calculated value makes sense because it is almost four times times the mass for 1 mole of aluminum. { "@context": "http://schema.org", { 1.9 x 1023atoms He x 1 mol He = 0.32 mol He. $6.022 x 1023). Enter your email address to follow this blog and receive notifications of new posts by email. Add highlights, virtual manipulatives, and more. of particles and moles with Avogadros number. ", Example \(\PageIndex{4}\): Generation of Aluminum Oxide. the number of oxygen atoms in 16 grams of oxygen? { NOTE: 10 pt QUIZ tomorrow, calculating formula AND molar mass (thats it!)
}, 15 Simply click it and you will be taken to a page where you can give a quick rating and leave a short comment for the product. Daily science Jan 14 Write a net ionic equation for the following: Aqueous solutions of calcium chloride and sodium carbonate form the precipitate calcium.  What is a mole? We can use that ability to answer stoichiometry questions in terms of the masses of a particular substance, in addition to moles. In a certain experiment, \(45.7 \: \text{g}\) of ammonium nitrate is decomposed. "@type": "ImageObject", Find: 1 mol \(\ce{NH_4NO_3} = 80.06 \: \text{g/mol}\), 1 mol \(\ce{N_2O} = 44.02 \: \text{g/mol}\), 1 mol \(\ce{H_2O} = 18.02 \: \text{g/mol}\).
What is a mole? We can use that ability to answer stoichiometry questions in terms of the masses of a particular substance, in addition to moles. In a certain experiment, \(45.7 \: \text{g}\) of ammonium nitrate is decomposed. "@type": "ImageObject", Find: 1 mol \(\ce{NH_4NO_3} = 80.06 \: \text{g/mol}\), 1 mol \(\ce{N_2O} = 44.02 \: \text{g/mol}\), 1 mol \(\ce{H_2O} = 18.02 \: \text{g/mol}\).
(LogOut/ The mass, number of moles, concentration or volume of a substance can be calculated easily if you learn two formula triangles. \(3.00 \: \cancel{\text{mol} \: \ce{CaCl_2}} \times \dfrac{110.98 \: \text{g} \: \ce{CaCl_2}}{1 \: \cancel{\text{mol} \: \ce{CaCl_2}}} = 333 \: \text{g} \: \ce{CaCl_2}\), \(1 \: \text{mol} \: \ce{H_2O} = 18.02 \: \text{g}\) H, \(108 \: \cancel{\text{g} \: \ce{H_2O}} \times \dfrac{1 \: \text{mol} \: \ce{H_2O}}{18.02 \: \cancel{\text{g} \: \ce{H_2O}}} = 5.99 \: \text{mol} \: \ce{H_2O}\), \(249\, \cancel{g\, AlCl_{3}}\times \dfrac{1\, \cancel{mol\, AlCl_{3}}}{133.33\, \cancel{g\, AlCl_{3}}}\times \dfrac{6\, mol\, HCl}{2\, \cancel{mol\, AlCl_{3}}}=5.60\, mol\, HCl\), Find a balanced equation that describes the reaction, Since each mole of oxygen produces twice as many moles of water, it makes sense that the produced amount is greater than the reactant amount. Our final answer is expressed to three significant figures. "name": "Calculate number of atoms in 2.5 mol Kr. ). A mole is a QUANTITY of whatever you are measuring 1 mole = 6.02 x of whatever you are measuring Just like 1 dozen =. 2.5 moles = atoms. What was the same? the number of H 2 O molecules in 18grams. "width": "1024" Dimensional analysis will allow you to calculate the mass of \(\ce{CaCl_2}\) that you should measure as show in Example \(\PageIndex{3}\). Collections of items include dozen, gross, and mole.
Thus, we can read this reaction as two moles of hydrogen react with one mole of oxygen to produce two moles of water.. atoms 2.5 mol Kr x 6.022x1023 atoms Kr = 1.5 x 1024 atoms 1 mol Kr "width": "1024" Introduction to the Mole Background When you buy eggs you usually ask for a _______ eggs. BUT it is SO large that the ONLY things we count with it are atoms and molecules! The representative particles can be atoms, molecules, or formula units of ionic compounds. Balanced chemical equations are balanced not only at the molecular level but also in terms of molar amounts of reactants and products. For example, this equation is also balanced if we write it as, The ratio of the coefficients is 4:2:4, which reduces to 2:1:2. We also established that 1 mol of Al has a mass of 26.98 g (Example). Record your data on the data table on the board. "@type": "ImageObject", "@context": "http://schema.org", }, 9 A mole is the same, no matter if it\u2019s counting eggs or atoms. The first step in this case is to convert the known mass into moles, using the substances molar mass as the conversion factor. ", "description": "6.02 x 1023 atoms = 1 mole x 1023 atoms = moles. "width": "1024" The Mole IV. A mole is the same, no matter if its counting eggs or atoms. Prepare a concept map and use the proper conversion factor. In such a conversion, we use the molar mass of a substance as a conversion factor to convert mole units into mass units (or, conversely, mass units into mole units).  Avogadros Law is that 1 mole of any gas occuplies the same volume under the same conditions and, at room temperature and pressure, 1 mole of gas is always 24 litres. Suppose we want to use larger numbers. "width": "1024"
Avogadros Law is that 1 mole of any gas occuplies the same volume under the same conditions and, at room temperature and pressure, 1 mole of gas is always 24 litres. Suppose we want to use larger numbers. "width": "1024" 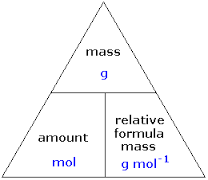 Each answer has three significant figures. "@context": "http://schema.org", Many problems of this type can be answered in this manner. }, 13 6.02x1023 atoms He. NOTE: 10 pt QUIZ tomorrow, calculating formula AND molar mass (thats it!). HW Review Lets put some problems on the board Moles and MassMake sure to show all your work! Practice Start The Mole and Avogadros Number WS A mole of dollars (each in thick) would reach over 7000 light-years! The mol Fe2O3 units cancel, leaving mol SO3 unit. 1 Chapter 7 Chemical Quantities 7.1 The Mole Basic Chemistry Copyright 2011 Pearson Education, Inc. "width": "1024"
Each answer has three significant figures. "@context": "http://schema.org", Many problems of this type can be answered in this manner. }, 13 6.02x1023 atoms He. NOTE: 10 pt QUIZ tomorrow, calculating formula AND molar mass (thats it!). HW Review Lets put some problems on the board Moles and MassMake sure to show all your work! Practice Start The Mole and Avogadros Number WS A mole of dollars (each in thick) would reach over 7000 light-years! The mol Fe2O3 units cancel, leaving mol SO3 unit. 1 Chapter 7 Chemical Quantities 7.1 The Mole Basic Chemistry Copyright 2011 Pearson Education, Inc. "width": "1024"
"@type": "ImageObject", Prepare two concept maps and use the proper conversion factor. You and your row will get a baggie.Find the mass of the contents. My students love these and I let them use them on tests. Calculate the mass of 3.00 moles of calcium chloride (CaCl2). Item Number Mass (g) Drill Leave your HW on the desk Take out a sheet of notebook paper.Chem Joke of the Day What does a monkey call barium with two sodium atoms? Formula triangle to find moles, concentration and volume. How many moles are present in 100. g of Al? "@type": "ImageObject", What is the number of carbon atoms in 12 grams of carbon? "width": "1024" Counting Units A pair refers to how many shoes? { "contentUrl": "https://slideplayer.com/slide/12860497/78/images/7/Hmmm%E2%80%A6+What+was+the+same+What+was+different.jpg", ", "description": "Make sure to show all your work! Calculate the mass of 0.25 moles of butane, Religious, moral and philosophical studies. Are you getting the free resources, updates, and special offers we send out every week in our teacher newsletter? We get exactly the same answer when combining all the math steps together as we do when we calculate one step at a time. "contentUrl": "https://slideplayer.com/slide/12860497/78/images/14/Practice+Start+The+Mole+and+Avogadro%E2%80%99s+Number+WS.jpg", Count the contents. As an example, consider the balanced chemical equation, \[Fe_2O_3 + 3SO_3 \rightarrow Fe_2(SO_4)_3\]. "name": "Item Number Mass (g)", The lesson?
"width": "1024" \[mass = number\,of\,moles \times formula\,mass\], \[\begin{array}{l} Our final answer is expressed to four significant figures. "width": "1024" "@context": "http://schema.org", To make this website work, we log user data and share it with processors. "name": "What is a mole A mole is a counting unit, like a dozen. If we have 3.59 mol of Fe2O3, how many grams of SO3 can react with it? "width": "1024" Students always have difficulty remembering if they need to multiply or divide (and by what?!) ", \(3.987 \: \cancel{\text{mol} \: \ce{Al}} \times \dfrac{26.98 \: \text{g} \: \ce{Al}}{1 \: \cancel{\text{mol} \: \ce{Al}}} = 107.6 \: \text{g} \: \ce{Al}\). What is a mole? Use a balanced chemical equation to determine molar relationships between substances. = 14.5g "description": "Chem Joke of the Day. The Earth was formed only 4,600,000,000 years ago! }, 11 Calculate number of atoms in 2.5 mol Kr.One mole = x 1023 atoms 2.5 moles = ? "contentUrl": "https://slideplayer.com/slide/12860497/78/images/12/Calculate+number+of+moles+for+1.9+x+1023+atoms+of+He..jpg", "description": "Mole. The counting unit of Chemistry. What is the percent composition of N and O in NO 2 ?
We can extend this technique even further. Change). What was different? Voila! "contentUrl": "https://slideplayer.com/slide/12860497/78/images/13/Mole+Triangle+We+can+convert+between+Mol+%EF%83%A0+g+and+back.jpg", For the reaction in which hydrogen and oxygen combine to make water, for example, we can construct the following ratios: \[\mathrm{\dfrac{2\: mol\: H_2}{1\: mol\: O_2}\: or\: \dfrac{1\: mol\: O_2}{2\: mol\: H_2}}\], \[\mathrm{\dfrac{2\: mol\: H_2O}{1\: mol\: O_2}\: or\: \dfrac{1\: mol\: O_2}{2\: mol\: H_2O}}\], \[\mathrm{\dfrac{2\: mol\: H_2}{2\: mol\: H_2O}\: or\: \dfrac{2\: mol\: H_2O}{2\: mol\: H_2}}\]. Using the formula triangle is straightforward. Share buttons are a little bit lower. "@context": "http://schema.org", "contentUrl": "https://slideplayer.com/slide/12860497/78/images/1/The+Mole+Triangle+Mole+%EF%83%A0+Gram+Conversions.jpg", Create a free website or blog at WordPress.com. However, the equation is balanced as long as the coefficients are in a 2:1:2 ratio. It would take over 19,100,000,000,000,000 years.
Now, we take this answer and convert it to grams of SO3, using the molar mass of SO3 as the conversion factor: \[\mathrm{10.77\: \bcancel{mol\: SO_3} \times \left( \dfrac{80.06\: g\: SO_3}{1\: \bcancel{ mol\: SO_3}} \right) =862\: g\: SO_3} \label{Eq3}\]. ?If I gave you one dollar a second, how long do you think it would take for me to give you a mole of dollars? Calculate the mass of 0.25 moles of butane \({C_4}{H_{10}}\). ", Recall that we can relate a molar amount to a mass amount using molar mass. This last triangle isnt for mole conversion, but its helpful to know just in case. "name": "Calculate number of moles for 1.9 x 1023 atoms of He. Conversions like this are possible for any substance, as long as the proper atomic mass, formula mass, or molar mass is known (or can be determined) and expressed in grams per mole. "contentUrl": "https://slideplayer.com/slide/12860497/78/images/4/HW+Review+Let%E2%80%99s+put+some+problems+on+the+board+Moles+and+Mass.jpg", We do this using the following sequence: Collectively, these conversions are called mole-mass calculations. ", { "@type": "ImageObject", \[2AlCl_3 + 3H_2O() Al_2O_3 + 6HCl(g) \nonumber\], Exercise \(\PageIndex{4}\): Generation of Aluminum Oxide. \(45.7 \: \text{g} \: \ce{NH_4NO_3} \times \dfrac{1 \: \text{mol} \: \ce{NH_4NO_3}}{80.06 \: \text{g} \: \ce{NH_4NO_3}} \times \dfrac{1 \: \text{mol} \: \ce{N_2O}}{1 \: \text{mol} \: \ce{NH_4NO_3}} \times \dfrac{44.02 \: \text{g} \: \ce{N_2O}}{1 \: \text{mol} \: \ce{N_2O}} = 25.1 \: \text{g} \: \ce{N_2O}\), \(45.7 \: \text{g} \: \ce{NH_4NO_3} \times \dfrac{1 \: \text{mol} \: \ce{NH_4NO_3}}{80.06 \: \text{g} \: \ce{NH_4NO_3}} \times \dfrac{2 \: \ce{H_2O}}{1 \: \text{mol} \: \ce{NH_4NO_3}} \times \dfrac{18.02 \: \text{g} \: \ce{H_2O}}{1 \: \text{mol} \: \ce{H_2O}} = 20.6 \: \text{g} \: \ce{H_2O}\), Exercise \(\PageIndex{6}\): Carbon Tetrachloride. Published byAlexandrina Mills "name": "Hmmm\u2026 What was the same What was different", Calculate number of moles for 1.9 x 1023 atoms of He.6.02 x 1023 atoms = 1 mole 18.06 x 1023 atoms = ?
"description": "1 dozen eggs = 12 eggs. "@context": "http://schema.org", "contentUrl": "https://slideplayer.com/slide/12860497/78/images/9/What+is+a+mole+A+mole+is+a+counting+unit%2C+like+a+dozen..jpg", Record your data on the data table on the board. Change), You are commenting using your Twitter account. The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. We can divide both sides of this expression by either side to get one of two possible conversion factors: \[\mathrm{\dfrac{1\: mol\: Al}{26.98\: g\: Al}\, and\, \dfrac{26.98\: g\: Al}{1\: mol\: Al}} \label{Eq1}\], The first conversion factor can be used to convert from mass to moles, and the second converts from moles to mass. }, 7 Formula triangle for density, mass and volume. "name": "Mole Triangle We can convert between Mol \uf0e0 g and back", How many moles are present in 108 grams of water? 6.022 x 1023 eggs! How many moles of ammonia are produced if 4.20 moles of hydrogen are reacted with an excess of nitrogen?
{ The convention for writing balanced chemical equations is to use the lowest whole-number ratio for the coefficients. The Mole Triangle Mole Gram ConversionsChemistry 3/12/14 MarisaAlviar-Agnew(Sacramento City College). Fill in your details below or click an icon to log in: You are commenting using your WordPress.com account. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units (depending on the type of substance). NOTE: 10 pt QUIZ tomorrow, calculating formula AND molar mass (that\u2019s it! Consider the following coefficients: \[12.044 \times 10^{23}\; H_2 + 6.022 \times 10^{23}\; O_2 12.044 \times 10^{23}\; H_2O\], These coefficients also have the ratio 2:1:2 (check it and see), so this equation is balanced. The Earth was formed only 4,600,000,000 years ago! ", (Hint: you will have to use the other conversion factor we obtained for aluminum.). "description": "Chemistry.
= 0.25 \times 58\\ What was the same? document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); on Different Formulas for Quantitative Chemistry and MoleConversion, Chapter 35: The Balance of Payments Problem, Chapter 36: Protection of the Environment, Different Formulas for Quantitative Chemistry and MoleConversion. "contentUrl": "https://slideplayer.com/slide/12860497/78/images/15/Exit+Ticket+How+many+bananas+are+in+a+mole+of+bananas.jpg", \[\ce{NH_4NO_3} \left( s \right) \rightarrow \ce{N_2O} \left( g \right) + 2 \ce{H_2O} \left( l \right) \nonumber\]. Teachers Pay Teachers is an online marketplace where teachers buy and sell original educational materials. If we start with a known mass of one substance in a chemical reaction (instead of a known number of moles), we can calculate the corresponding masses of other substances in the reaction. "contentUrl": "https://slideplayer.com/slide/12860497/78/images/2/Drill+Leave+your+HW+on+the+desk+Take+out+a+sheet+of+notebook+paper..jpg", We have established that a balanced chemical equation is balanced in terms of moles as well as atoms or molecules. "description": "mol \uf0e0 particle and back. Simply cover whatever part of the triangle you are trying to work out.
Hmmm What was the same?
}, 10 What does a monkey call barium with two sodium atoms? Both can be used to solve problems that would be hard to do by eye.. Thus, in a two-step process, we find that 862 g of SO3 will react with 3.59 mol of Fe2O3. Would you like a mole of $? The same two-step problem can also be worked out in a single line, rather than as two separate steps, as follows: \[ 3.59 \cancel{\, mol \, Fe_2O_3} \times \underbrace{\left( \dfrac{ 3 \bcancel{ \, mol\, SO_3}}{ 1 \cancel{\, mol\, Fe_2O_3}} \right)}_{\text{converts to moles of SO}_3} \times \underbrace{ \left( \dfrac{ 80.06 {\, g \, SO_3}}{ 1 \, \bcancel{ mol\, SO_3}} \right)}_{\text{converts to grams of SO}_3} = 862\, g\, SO_3\]. How. Legal. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. How many moles of oxygen react with hydrogen to produce 27.6 mol of H2O? }, 6 "description": "Find the mass of the contents. This relationship is frequently used in the laboratory. The formula we need to use, given by covering up number of moles is: \[number\,of\,moles = \frac{{mass}}{{formula\,mass}}\]. 5.4: Molar Mass- Mole-to-Mass and Mass-to-Mole Conversions, [ "article:topic", "showtoc:no", "transcluded:yes", "source[1]-chem-152164" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FSacramento_City_College%2FSCC%253A_CHEM_330_-_Adventures_in_Chemistry_(Alviar-Agnew)%2F05%253A_Chemical_Accounting%2F5.04%253A_Molar_Mass-_Mole-to-Mass_and_Mass-to-Mole_Conversions, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\), Mole and Mass Relationships in Chemical Equations, Molar Relationships in Chemical Equations, status page at https://status.libretexts.org, Identify the "given"information and what the problem is asking you to "find. "@type": "ImageObject", These four triangles remind students what needs to be divided or multiplied when converting to moles, grams, molar mass, number of molecules, volume, or molarity. Objectives IWBAT Calculate formula or molar mass.Use the mole triangle to convert from moles to grams, moles to particles, or moles to liters. How many moles are present in 25 g of calcium carbonate? Write down the formula for the substance, and calculate the mass of that ONE MOLE. "@context": "http://schema.org", \[\ce{CH4 (g) + 4 Cl2 (g) CCl2 (l) + 4 HCl (l) }\]. "name": "Objectives IWBAT Calculate formula or molar mass. moles 1.9 x 1023atoms He x mol He = 0.32 mol He 6.02x1023 atoms He ", "@type": "ImageObject", Then using the molar mass of SO3 as a conversion factor, we determine the mass that this number of moles of SO3 has. Sequentially, the process is as follows: This three-part process can be carried out in three discrete steps or combined into a single calculation that contains three conversion factors. { Figure \(\PageIndex{1}\) is a chart for determining what conversion factor is needed, and Figure \(\PageIndex{2}\) is a flow diagram for the steps needed to perform a conversion. {
HW: The Mole and Avogadro\u2019s Number (back of WS)", Reported resources will be reviewed by our team. { "width": "1024" The result corresponds to the 3:2 ratio of hydrogen to ammonia from the balanced equation. A mole of dollars (each in thick) would reach over 7000 light-years! Change), You are commenting using your Facebook account. Find the mass of each of the products formed. If I gave you one dollar a second, how long do you think it would take for me to give you a mole of dollars? \(\mathrm{\cancel{27.6\: mol\: H_2O}\times\dfrac{1\: mol\: O_2}{\cancel{2\: mol\: H_2O}}=13.8\: mol\: O_2}\). Find the Cu and the Fe. "contentUrl": "https://slideplayer.com/slide/12860497/78/images/10/Would+you+like+a+mole+of+%24.jpg", First, we construct the appropriate molar ratio, determined from the balanced chemical equation, to calculate the number of moles of SO3 needed. Using the mole-mass calculation sequence, we can determine the required mass of SO3 in two steps. A mole is the same, no matter if its counting eggs or atoms. (LogOut/ How many moles of HCl will be produced when 249 g of AlCl3 are reacted according to this chemical equation? Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. The Mole. }. Pre-made digital activities. { Thank you! "@type": "ImageObject", Chemistry An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition Copyright 2012 by Pearson Education, Inc. Chapter 5 Chemical. . { Chem Instructions Before class starts, get a piece of paper and title it Ch 11 Notes The Mole. Find the Cu and the Fe. }, 8 "description": "Item Number Mass (g)", What is a mole? { Given the following balanced chemical equation,\[\ce{C5H12 + 8O2 5CO2 + 6H2O}\]how many moles of H, Balance the following unbalanced equation and determine how many moles of H. Calculations involving conversions between moles of a substance and the mass of that substance are described. The Earth was formed only 4,600,000,000 years ago! Methane can react with elemental chlorine to make carbon tetrachloride (\(\ce{CCl_4}\)). "description": "What do we call that number Look back at the drill. , Be the first to know about my new discounts, freebies and product launches, Look for the green star next to my store logo and click it to become a follower. Count the contents. Intro Questions: Write these questions down on a separate sheet of paper so that at the end of class you can answer the questions and hand your responses.
BUT it is SO large that the ONLY things we count with it are atoms and molecules! A mole of dollars (each in thick) would reach over 7000 light-years! "contentUrl": "https://slideplayer.com/slide/12860497/78/images/8/The+counting+unit+of+Chemistry.jpg", Look back at the drill. Take out a sheet of notebook paper. Find the Cu and the Fe. A mole is a counting unit, like a dozen.1 dozen eggs = 12 eggs 1 mole eggs = ? BUT it is SO large that the ONLY things we count with it are atoms and molecules!