 The principal product in this case is R-Nuc. Under certain conditions nucleophilic substitutions may occur, via other mechanisms such as those described in the nucleophilic aromatic substitution article. Common examples include. Besides SN1 and SN2, other mechanisms are known, although they are less common. Common examples include: An example of a substitution reaction taking place by a so-called borderline mechanism as originally studied by Hughes and Ingold[6] is the reaction of 1-phenylethyl chloride with sodium methoxide in methanol. A nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). It is also due to this carbocation intermediate that the product does not have to have inversion. The most general form for the reaction may be given as. sk:Nukleofiln substitcia, Nucleophilic substitution at saturated carbon centres, Nucleophilic substitution at unsaturated carbon centres. When the substitution occurs at the carbonyl group, the acyl group may undergo nucleophilic acyl substitution. SN2 occurs where the central carbon atom is easily accessible to the nucleophile. Aprotic solvents do not add protons (H+ ions) into solution; if protons were present in SN2 reactions, they would react with the nucleophile and severely limit the reaction rate. https://en.wikipedia.org/w/index.php?title=Nucleophilic_substitution&oldid=1063867142, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, Never unless additional stabilising groups present, Good unless a hindered nucleophile is used, Common, especially with basic nucleophiles, This page was last edited on 5 January 2022, at 09:25. 1.) Nucleophilic substitution reactions are commonplace in organic chemistry, and they can be broadly categorised as taking place at an aliphatic (saturated) carbon or at (less often) an aromatic or other unsaturated carbon centre. SN2 - Watch out of the steric hindrance blocking the nucleophile. Since there is an intermediate that actually contains a positive charge, bulky groups attached are going to help stabilize the charge on the carbocation through resonance and distribution of charge. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. The principal product in this case is R-Nuc. Initially, the rate of the nucleophilic substitution was a little puzzling as the rate followed the pattern: The reaction kinetics changed from second order to first order. With allylic halides or sulphonates, for example, the nucleophile may attack at the unsaturated carbon in place of the carbon bearing the leaving group. A graph showing the relative reactivities of the different alkyl halides towards SN1 and SN2 reactions.
The principal product in this case is R-Nuc. Under certain conditions nucleophilic substitutions may occur, via other mechanisms such as those described in the nucleophilic aromatic substitution article. Common examples include. Besides SN1 and SN2, other mechanisms are known, although they are less common. Common examples include: An example of a substitution reaction taking place by a so-called borderline mechanism as originally studied by Hughes and Ingold[6] is the reaction of 1-phenylethyl chloride with sodium methoxide in methanol. A nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). It is also due to this carbocation intermediate that the product does not have to have inversion. The most general form for the reaction may be given as. sk:Nukleofiln substitcia, Nucleophilic substitution at saturated carbon centres, Nucleophilic substitution at unsaturated carbon centres. When the substitution occurs at the carbonyl group, the acyl group may undergo nucleophilic acyl substitution. SN2 occurs where the central carbon atom is easily accessible to the nucleophile. Aprotic solvents do not add protons (H+ ions) into solution; if protons were present in SN2 reactions, they would react with the nucleophile and severely limit the reaction rate. https://en.wikipedia.org/w/index.php?title=Nucleophilic_substitution&oldid=1063867142, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, Never unless additional stabilising groups present, Good unless a hindered nucleophile is used, Common, especially with basic nucleophiles, This page was last edited on 5 January 2022, at 09:25. 1.) Nucleophilic substitution reactions are commonplace in organic chemistry, and they can be broadly categorised as taking place at an aliphatic (saturated) carbon or at (less often) an aromatic or other unsaturated carbon centre. SN2 - Watch out of the steric hindrance blocking the nucleophile. Since there is an intermediate that actually contains a positive charge, bulky groups attached are going to help stabilize the charge on the carbocation through resonance and distribution of charge. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. The principal product in this case is R-Nuc. Initially, the rate of the nucleophilic substitution was a little puzzling as the rate followed the pattern: The reaction kinetics changed from second order to first order. With allylic halides or sulphonates, for example, the nucleophile may attack at the unsaturated carbon in place of the carbon bearing the leaving group. A graph showing the relative reactivities of the different alkyl halides towards SN1 and SN2 reactions.
In the intermediate step, the nucleophile is 185 degrees from the leaving group and the stereochemistry is inverted as the nucleophile bonds to make the product. Unimolecular Nucleophilic Substitution does not Exist! he:
Less often, they may attack an aromatic or unsaturated carbon. The SNi mechanism is observed in reactions of thionyl chloride with alcohols, and it is similar to SN1 except that the nucleophile is delivered from the same side as the leaving group. Cookies help us deliver our services. SN2 - methyl > primary > secondary (you want the LG to be less crowded), SN1 - tertiary > secondary ( you want the LG to be more crowded). In this case, halogens are going to be the best leaving groups, while compounds such as amines, hydrogen, and alkanes are going to be quite poor leaving groups. This may be seen in the reaction of 1-chloro-2-butene with sodium hydroxide to give a mixture of 2-buten-1-ol and 1-buten-3-ol: The Sn1CB mechanism appears in inorganic chemistry. The SNi mechanism is observed in reactions of thionyl chloride with alcohols, and it is similar to SN1 except that the nucleophile is delivered from the same side as the leaving group. This is the normal mode of substitution with carboxylic acid derivatives such as acyl chlorides, esters and amides. Simultaneously, the leaving group (LG) departs with an electron pair. Like SN2 reactions, there are quite a few factors that affect the reaction rate of SN1 reactions. it:Sostituzione nucleofila  SN1 - Stabilizing the carbocation formed. The nucleophile can attack from the top or the bottom and therefore create a racemic product. Instead of having two concentrations that affect the reaction rate, there is only one, substrate. The rate equation for this would be Rate=k[Sub]. This type of mechanism is called an SN1' or SN2' reaction (depending on the kinetics). SN2 - polar Aprotic ( no O-H or N-H bonds) a concerted reaction). SN1 reactions tend to be important when the central carbon atom of the substrate is surrounded by bulky groups, both because such groups interfere sterically with the SN2 reaction (discussed above) and because a highly substituted carbon forms a stable carbocation.
SN1 - Stabilizing the carbocation formed. The nucleophile can attack from the top or the bottom and therefore create a racemic product. Instead of having two concentrations that affect the reaction rate, there is only one, substrate. The rate equation for this would be Rate=k[Sub]. This type of mechanism is called an SN1' or SN2' reaction (depending on the kinetics). SN2 - polar Aprotic ( no O-H or N-H bonds) a concerted reaction). SN1 reactions tend to be important when the central carbon atom of the substrate is surrounded by bulky groups, both because such groups interfere sterically with the SN2 reaction (discussed above) and because a highly substituted carbon forms a stable carbocation. 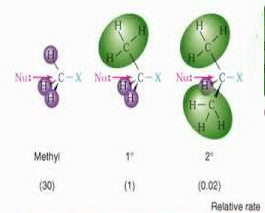 The SN1 and SN2 reactions are influenced by different factors, SN1 reactivity rates follow the trend CH3X < primary < secondary < tertiary, SN2 reactivity rates follow the trend CH3X > primary > secondary > tertiary. With allylic halides or sulphonates, for example, the nucleophile may attack at the unsaturated carbon in place of the carbon bearing the leaving group. Under certain conditions nucleophilic substitutions may occur, via other mechanisms such as those described in the nucleophilic aromatic substitution article. How does temperature affect SN1 and SN2 reactions? The reaction rate is found to the sum of SN1 and SN2 components with 61% (3,5 M, 70C) taking place by the latter. Why do haloalkanes undergo nucleophilic substitution? In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of substitution reaction in which an "electron rich" nucleophile selectively bonds with or attacks the positive charge of a group or atom called the leaving group; rarely referred to as an electrophobe. The total reactivity is the sum of the two rates. Nucleophiles often attack a saturated aliphatic carbon. When the substitution occurs at the carbonyl group, the acyl group may undergo nucleophilic acyl substitution. https://www.wikidoc.org/index.php?title=Nucleophilic_substitution&oldid=720094, Creative Commons Attribution/Share-Alike License. SN1 - Racemization, Substitution and elimination usually compete and are both formed in the product. The molecule that contains the electrophile and the leaving functional group is called the substrate.[1][2]. The electron pair (:) from the nucleophile (Nuc) attacks the substrate (R-LG) forming a new bond, while the leaving group (LG) departs with an electron pair. First of all, the 2 in SN2 implies that there are two concentrations of substances that affect the rate of reaction: substrate (Sub) and nucleophile. Competing mechanisms exist.[7][8]. What are common mistakes students make with nucleophilic substitutions? Besides SN1 and SN2, other mechanisms are known, although they are less common. [4], In the SN2 reaction, the addition of the nucleophile and the elimination of leaving group take place simultaneously (i.e. In organometallic chemistry the nucleophilic abstraction reaction occurs with a nucleophilic substitution mechanism. Solvent J. P. Clayden, N. Greeves, S. Warren, P. D. Wothers, This page was last edited 20:11, 4 September 2012 by wikidoc user. It does not matter if the hydrogens from the protic solvent react with the nucleophile since the nucleophile is not involved in the rate determining step. By contrast the SN1 reaction involves two steps. Nucleophilic substitutions can be accompanied by an allylic rearrangement as seen in reactions such as the Ferrier rearrangement.
The SN1 and SN2 reactions are influenced by different factors, SN1 reactivity rates follow the trend CH3X < primary < secondary < tertiary, SN2 reactivity rates follow the trend CH3X > primary > secondary > tertiary. With allylic halides or sulphonates, for example, the nucleophile may attack at the unsaturated carbon in place of the carbon bearing the leaving group. Under certain conditions nucleophilic substitutions may occur, via other mechanisms such as those described in the nucleophilic aromatic substitution article. How does temperature affect SN1 and SN2 reactions? The reaction rate is found to the sum of SN1 and SN2 components with 61% (3,5 M, 70C) taking place by the latter. Why do haloalkanes undergo nucleophilic substitution? In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of substitution reaction in which an "electron rich" nucleophile selectively bonds with or attacks the positive charge of a group or atom called the leaving group; rarely referred to as an electrophobe. The total reactivity is the sum of the two rates. Nucleophiles often attack a saturated aliphatic carbon. When the substitution occurs at the carbonyl group, the acyl group may undergo nucleophilic acyl substitution. https://www.wikidoc.org/index.php?title=Nucleophilic_substitution&oldid=720094, Creative Commons Attribution/Share-Alike License. SN1 - Racemization, Substitution and elimination usually compete and are both formed in the product. The molecule that contains the electrophile and the leaving functional group is called the substrate.[1][2]. The electron pair (:) from the nucleophile (Nuc) attacks the substrate (R-LG) forming a new bond, while the leaving group (LG) departs with an electron pair. First of all, the 2 in SN2 implies that there are two concentrations of substances that affect the rate of reaction: substrate (Sub) and nucleophile. Competing mechanisms exist.[7][8]. What are common mistakes students make with nucleophilic substitutions? Besides SN1 and SN2, other mechanisms are known, although they are less common. [4], In the SN2 reaction, the addition of the nucleophile and the elimination of leaving group take place simultaneously (i.e. In organometallic chemistry the nucleophilic abstraction reaction occurs with a nucleophilic substitution mechanism. Solvent J. P. Clayden, N. Greeves, S. Warren, P. D. Wothers, This page was last edited 20:11, 4 September 2012 by wikidoc user. It does not matter if the hydrogens from the protic solvent react with the nucleophile since the nucleophile is not involved in the rate determining step. By contrast the SN1 reaction involves two steps. Nucleophilic substitutions can be accompanied by an allylic rearrangement as seen in reactions such as the Ferrier rearrangement.
simple:Nucleophilic substitution mk: The two main mechanisms were the SN1 reaction and the SN2 reaction, where S stands for substitution, N stands for nucleophilic, and the number represents the kinetic order of the reaction. / N.S.Imyanitov. Since this reaction occurs in one step, steric effects drive the reaction speed. By using our services, you agree to our use of cookies. A final factor that affects reaction rate is nucleophilicity; the nucleophile must attack an atom other than a hydrogen. around the world, Nucleophilic Substitution Reactions (SN1 and SN2) and Elimination Reactions (E1 and E2). da:Nukleofil substitution There are many reactions in organic chemistry that involve this type of mechanism. SN1 - polar Protic (at least one O-H or N-H bonds), 2.) Also, because the intermediate is partially bonded to the nucleophile and leaving group, there is no time for the substrate to rearrange itself: the nucleophile will bond to the same carbon that the leaving group was attached to. See all questions in SN1 and SN2 Reactions.
In the SN2 reaction, the addition of the nucleophile and the elimination of leaving group take place simultaneously. There are many reactions in organic chemistry involve this type of mechanism. Explain nucleophilic substitution of alkyl halides? [1], In 1935, Edward D. Hughes and Sir Christopher Ingold studied nucleophilic substitution reactions of alkyl halides and related compounds. Nucleophilic substitution via the SN1 or SN2 mechanism does not generally occur with vinyl or aryl halides or related compounds. [3], In 1935, Edward D. Hughes and Sir Christopher Ingold studied nucleophilic substitution reactions of alkyl halides and related compounds.
This means that the better the leaving group, the faster the reaction rate. A general rule for what makes a good leaving group is the weaker the conjugate base, the better the leaving group. This type of mechanism is called an SN1' or SN2' reaction (depending on the kinetics). The most general form of the reaction may be given as the following: The electron pair (:) from the nucleophile (Nuc) attacks the substrate (R-LG) and bonds with it. de:Nukleophile Substitution id:Substitusi nukleofilik The two main mechanisms are the SN1 reaction and the SN2 reaction. How does an SN2 reaction affect stereochemistry?
Answer the exercises from the book over and over again and it'll make sense. Nucleophilic substitution reactions are common in organic chemistry. This is the normal mode of substitution with carboxylic acid derivatives such as acyl chlorides, esters and amides. Nucleophilic substitution via the SN1 or SN2 mechanism does not generally occur with vinyl or aryl halides or related compounds. SN1: Racemization, 7908 views How does concentration affect SN2 reactions?
However, one will be the major and the other will be the minor product. It is important to use a protic solvent, water and alcohols, since an aprotic solvent could attack the intermediate and cause unwanted product. This may be seen in the reaction of 1-chloro-2-butene with sodium hydroxide to give a mixture of 2-buten-1-ol and 1-buten-3-ol: The Sn1CB mechanism appears in inorganic chemistry. SN2 - Inversion ( you do a backside attack, since LG is blocking the frontside) SN2 occurs when the central carbon atom is easily accessible to the nucleophile.[5]. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is OH and the leaving group is Br. S stands for chemical substitution, N stands for nucleophilic, and the number represents the kinetic order of the reaction.[2]. In this case, tertiary carbocation will react faster than a secondary which will react much faster than a primary. For a SN2 reaction, an aprotic solvent is best, such as acetone, DMF, or DMSO. By contrast the SN1 reaction involves two steps. Side Note : Substrate ( Leaving group (LG) attached to the carbon is) As SN2 reactions were affected by sterics, SN1 reactions are determined by bulky groups attached to the carbocation. The rate equation for this reaction would be Rate=k[Sub][Nuc]. It is confusing at first but practice makes perfect. How does concentration affect SN1 reactions? Nucleophilic substitutions can be accompanied by an allylic rearrangement as seen in reactions such as the Ferrier rearrangement. SN1 reactions tend to be important when the central carbon atom of the substrate is surrounded by bulky groups, both because such groups interfere sterically with the SN2 reaction (discussed above) and because a highly substituted carbon forms a stable carbocation. Stereochemistry: Since the rate of a reaction is only determined by its slowest step, the rate at which the leaving group "leaves" determines the speed of the reaction. They proposed that there were two main mechanisms at work, both of them competing with each other. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br, under alkaline conditions, where the attacking nucleophile is the OH and the leaving group is Br-. Also see Table 1. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. In SN2 reactions, there are a few conditions that affect the rate of the reaction. They proposed that there were two main mechanisms at work, both of them competing with each other.