Victorov, A. I. and Koroleva, S. V., Modeling of the effects of ion specificity on the onset and growth of ionic micelles in a solution of simple salts. The cmc and the alpha values increased with the increase in the number of headgroups of the surfactant. The https:// ensures that you are connecting to the If you are an author contributing to an RSC publication, you do not need to request permission
Colloids and Surfaces A, 71, p. 39-64 (1993). <>>>
v@Pi9boT]k.`!im0 &,s?:D[. Molecular dynamics study of non-nucleoside reverse transcriptase inhibitor 4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]benzonitrile (TMC278/rilpivirine) aggregates: correlation between amphiphilic properties of the drug and oral bioavailability. Byrd, R. H., Gilbert, J. C. and Nocedal, J., A trust region method based on interior point techniques for nonlinear programming. T s, sph to access the full features of the site or access our, Heilongjiang Academy of Sciences Institute of Advanced Technology, Harbin, China, Harbin FRP Research Institute, Harbin, Heilongjiang 150029, China, Creative Commons Attribution-NonCommercial 3.0 Unported Licence, Copyright
Pressure (Pa) Water The surface chemical parameters and thermodynamics parameters were obtained. 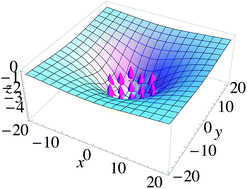 official website and that any information you provide is encrypted Coarse-grained molecular dynamics simulation of the aggregation properties of multiheaded cationic surfactants in water. provided that the correct acknowledgement is given and it is not used for commercial purposes. )Pe}>?]}>}|%W/BsQ,' KM /l/af+F5~C(|`* Fetching data from CrossRef. HHS Vulnerability Disclosure, Help Journal of Physical Chemistry, B, 116, p. 6443-54 (2012). Langmuir, 26(19), p. 15177-15191 (2010). Unable to load your collection due to an error, Unable to load your delegates due to an error. Puvvada, S. and Blankschtein, D., Molecular-thermodynamic approach to predict micellization, phase behavior and phase separation of micellar solutions.
sharing sensitive information, make sure youre on a federal Temperature (K) To request permission to reproduce material from this article in a commercial publication,
This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
official website and that any information you provide is encrypted Coarse-grained molecular dynamics simulation of the aggregation properties of multiheaded cationic surfactants in water. provided that the correct acknowledgement is given and it is not used for commercial purposes. )Pe}>?]}>}|%W/BsQ,' KM /l/af+F5~C(|`* Fetching data from CrossRef. HHS Vulnerability Disclosure, Help Journal of Physical Chemistry, B, 116, p. 6443-54 (2012). Langmuir, 26(19), p. 15177-15191 (2010). Unable to load your collection due to an error, Unable to load your delegates due to an error. Puvvada, S. and Blankschtein, D., Molecular-thermodynamic approach to predict micellization, phase behavior and phase separation of micellar solutions.
sharing sensitive information, make sure youre on a federal Temperature (K) To request permission to reproduce material from this article in a commercial publication,
This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Bookshelf Rusanov, A. I., The wonderful world of micelles. Moreira, L. A. and Firoozabadi, A., Molecular thermodynamic modeling of specific ion effects on micellization of ionic surfactants. Nagarajan, R. and Ruckenstein, E., Theory of surfactant self -assembly: A predictive molecular thermodynamic approach. MOLECULAR THERMODYNAMICS OF MICELLIZATION: MICELLE SIZE DISTRIBUTIONS AND GEOMETRY TRANSITIONS, Micelle surface area per surfactant at a distance, Superficial area of the micelle of aggregation number, Length of the cylindrical part of the micelle (m), Radius of the cylindrical part of the micelle (m), Radius of the spherical part of the micelle (m), Volume of the micelle of aggregation number. Langmuir, 7(3), p. 2934-2969 (1991). Journal of Molecular Liquids, 198, p. 286-291 (2014). Colloids Surf B Biointerfaces. 2020 Nov 24;21(23):8912. doi: 10.3390/ijms21238912. Bouchemal K, Agnely F, Koffi A, Djabourov M, Ponchel G. J Mol Recognit. SA 68502, CEP: 21941-972, Rio de Janeiro, RJ - Brazil. The calculation results of thermodynamic parameters show that both adsorption and micellization processes of [Cnmpy][Br] are spontaneous; the enthalpy of [C12mpy][Br] is negative at 293.15 K and becomes negative with temperature increasing. Careers. The micellization and surface activity properties of long-chain pyridinium ionic liquids n-alkyl-3-methylpyridinium bromide ([Cnmpy][Br], n: the carbon numbers of hydrophobic tails, n = 12, 14, 16) in aqueous solution were systematically investigated through electronic conductivity measurement, surface tension, and ultraviolet-absorption spectra. Would you like email updates of new search results? Journal of Colloid and Interface Science, 306, p. 166-74 (2007). What can isothermal titration microcalorimetry experiments tell us about the self-organization of surfactants into micelles? Thermodynamics studies on tyrosine-hydantoin drug-cetyltrimethylammonium bromide mixed micellar system. Mathematical Programing, 89(1), p. 149-185 (2000). Read more about how to correctly acknowledge RSC content. 2009 Oct 8;52(19):5896-905. doi: 10.1021/jm900282z. Epub 2010 Sep 20. Subscripts Critical micelle concentrations of the sodium salts. c, cyl
Radius of the spherical part of the micelle (m) <>
(3), Stay informed of issues for this journal through your RSS reader, Text Cylindrical. Langmuir, 18(01), p. 31-38 (2002). J Phys Chem B. If you want to reproduce the whole
<>
this article in other publications, without requesting further permission from the RSC,
The DeltaG(m) values progressively became less negative with the increase in the number of headgroups. The effects of inorganic salts (LiBr, NaBr, MgBr2), organic alcohols (C2H5OH, C3H7OH, C4H9OH, C5H11OH) and temperature on the critical micelle concentration (CMC) values of [Cnmpy][Br] aqueous solutions were also investigated. Langmuir, 28(3), p. 1738-1752 (2012). You can use material from
For [C14mpy][Br] and [C16mpy][Br] this transition occurs at 288.15 K and the micellization process is entropy-driven in the investigated temperature range. Accessibility Length of the cylindrical part of the micelle (m) Escola de Qumica, Universidade Federal do Rio de Janeiro, CEP: 21941-909, Rio de Janeiro - RJ, Brazil. Langmuir, 30(22), p. 6373-6383 (2014). Clearance Center request page.
Surfactant Langmuir, 25(20), p. 12101-12113 (2009). Bethesda, MD 20894, Web Policies D. Fu, X. Gao, B. Huang, J. Wang, Y. DOI: 10.1039/C9RA04226A. Sun, W. Zhang, K. Kan, X. Zhang, Y. Xie and X. Sui,
<>
(3), Stay informed of issues for this journal through your RSS reader, Text Cylindrical. Langmuir, 18(01), p. 31-38 (2002). J Phys Chem B. If you want to reproduce the whole
<>
this article in other publications, without requesting further permission from the RSC,
The DeltaG(m) values progressively became less negative with the increase in the number of headgroups. The effects of inorganic salts (LiBr, NaBr, MgBr2), organic alcohols (C2H5OH, C3H7OH, C4H9OH, C5H11OH) and temperature on the critical micelle concentration (CMC) values of [Cnmpy][Br] aqueous solutions were also investigated. Langmuir, 28(3), p. 1738-1752 (2012). You can use material from
For [C14mpy][Br] and [C16mpy][Br] this transition occurs at 288.15 K and the micellization process is entropy-driven in the investigated temperature range. Accessibility Length of the cylindrical part of the micelle (m) Escola de Qumica, Universidade Federal do Rio de Janeiro, CEP: 21941-909, Rio de Janeiro - RJ, Brazil. Langmuir, 30(22), p. 6373-6383 (2014). Clearance Center request page.
Surfactant Langmuir, 25(20), p. 12101-12113 (2009). Bethesda, MD 20894, Web Policies D. Fu, X. Gao, B. Huang, J. Wang, Y. DOI: 10.1039/C9RA04226A. Sun, W. Zhang, K. Kan, X. Zhang, Y. Xie and X. Sui,
Colloids and Surfaces A: Physicochemical and Engineering Aspects, 412, p. 90-95 (2012).
3 0 obj Complementary use of simulations and molecular-thermodynamic theory to model micellization. Part I. Conventional (pH-Insensitive) surfactants.
The CMC values assumed a trend of decreasing and then increasing with the increase of temperature. (English), https://doi.org/10.1590/0104-6632.20160333s20150129. Kamranfar, P. and Jamialahmadi M., Effect of surfactant micelle shape transition on the microemulsion viscosity and its application in enhanced oil recovery processes. These studies suggest that the micelles are more hydrated with multiheaded surfactants and the micropolarity of micelles increases with the increase in the number of headgroups. Neves, A. C. S., Valente, A. J. M., Burrows, H. D., Ribeiro, A. C. F., Lobo, V. M. M., Effect of terbium(III) chloride on the micellization properties of sodium decyl and dodecyl-sulfate solutions. Microcalorimetric and conductivity studies with micelles prepared from multi-headed pyridinium surfactants. w Number of molecules of i government site. <>/XObject<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI] >>/MediaBox[ 0 0 612 792] /Contents 4 0 R/Group<>/Tabs/S/StructParents 0>> Journal of Chemical Society, p. 579-586 (1956). P You do not have JavaScript enabled. E-mail: tavares@eq.ufrj.br. Free energy of micellization (J) endobj Kennedy, J. and Eberhart, R., Particle swarm optimization. Tiwary LK, Mandal A, Alam MS, Thennarasu S, Mandal AB. Creative Commons Attribution-NonCommercial 3.0 Unported Licence. and transmitted securely. Langmuir, 30(12), p. 3387-3396 (2014). Federal government websites often end in .gov or .mil. Negative DeltaH(m) values increased from CTAB to h = 1 to h = 2 and decreased for h = 3 whereas DeltaS(m) values decreased with increase in the number of headgroups. 8600 Rockville Pike Volume of the surfactant tail (m3) please go to the Copyright Clearance Center request page. Pf Packing factor J. Chem. The critical micellar concentrations (cmc's) and the degrees of counterion dissociation (alpha) of micelles of these surfactants were also determined by conductometry. Rs
This article is licensed under a
Velzquez, M. M. and Lpez-Daz, D., Variation of the critical micelle concentration with surfactant structure: A simple method to analyze the role of attractive - repulsive forces on micellar association. Journal of Chemical Society of Pakistan, 30(2), p. 186-191 (2008). Frenkel YV, Gallicchio E, Das K, Levy RM, Arnold E. J Med Chem. kankan.has@foxmail.com, xc_zhang.has@hotmail.com, b endobj
 official website and that any information you provide is encrypted Coarse-grained molecular dynamics simulation of the aggregation properties of multiheaded cationic surfactants in water. provided that the correct acknowledgement is given and it is not used for commercial purposes. )Pe}>?]}>}|%W/BsQ,' KM /l/af+F5~C(|`* Fetching data from CrossRef. HHS Vulnerability Disclosure, Help Journal of Physical Chemistry, B, 116, p. 6443-54 (2012). Langmuir, 26(19), p. 15177-15191 (2010). Unable to load your collection due to an error, Unable to load your delegates due to an error. Puvvada, S. and Blankschtein, D., Molecular-thermodynamic approach to predict micellization, phase behavior and phase separation of micellar solutions.
sharing sensitive information, make sure youre on a federal Temperature (K) To request permission to reproduce material from this article in a commercial publication,
This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
official website and that any information you provide is encrypted Coarse-grained molecular dynamics simulation of the aggregation properties of multiheaded cationic surfactants in water. provided that the correct acknowledgement is given and it is not used for commercial purposes. )Pe}>?]}>}|%W/BsQ,' KM /l/af+F5~C(|`* Fetching data from CrossRef. HHS Vulnerability Disclosure, Help Journal of Physical Chemistry, B, 116, p. 6443-54 (2012). Langmuir, 26(19), p. 15177-15191 (2010). Unable to load your collection due to an error, Unable to load your delegates due to an error. Puvvada, S. and Blankschtein, D., Molecular-thermodynamic approach to predict micellization, phase behavior and phase separation of micellar solutions.
sharing sensitive information, make sure youre on a federal Temperature (K) To request permission to reproduce material from this article in a commercial publication,
This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Bookshelf Rusanov, A. I., The wonderful world of micelles. Moreira, L. A. and Firoozabadi, A., Molecular thermodynamic modeling of specific ion effects on micellization of ionic surfactants. Nagarajan, R. and Ruckenstein, E., Theory of surfactant self -assembly: A predictive molecular thermodynamic approach. MOLECULAR THERMODYNAMICS OF MICELLIZATION: MICELLE SIZE DISTRIBUTIONS AND GEOMETRY TRANSITIONS, Micelle surface area per surfactant at a distance, Superficial area of the micelle of aggregation number, Length of the cylindrical part of the micelle (m), Radius of the cylindrical part of the micelle (m), Radius of the spherical part of the micelle (m), Volume of the micelle of aggregation number. Langmuir, 7(3), p. 2934-2969 (1991). Journal of Molecular Liquids, 198, p. 286-291 (2014). Colloids Surf B Biointerfaces. 2020 Nov 24;21(23):8912. doi: 10.3390/ijms21238912. Bouchemal K, Agnely F, Koffi A, Djabourov M, Ponchel G. J Mol Recognit. SA 68502, CEP: 21941-972, Rio de Janeiro, RJ - Brazil. The calculation results of thermodynamic parameters show that both adsorption and micellization processes of [Cnmpy][Br] are spontaneous; the enthalpy of [C12mpy][Br] is negative at 293.15 K and becomes negative with temperature increasing. Careers. The micellization and surface activity properties of long-chain pyridinium ionic liquids n-alkyl-3-methylpyridinium bromide ([Cnmpy][Br], n: the carbon numbers of hydrophobic tails, n = 12, 14, 16) in aqueous solution were systematically investigated through electronic conductivity measurement, surface tension, and ultraviolet-absorption spectra. Would you like email updates of new search results? Journal of Colloid and Interface Science, 306, p. 166-74 (2007). What can isothermal titration microcalorimetry experiments tell us about the self-organization of surfactants into micelles? Thermodynamics studies on tyrosine-hydantoin drug-cetyltrimethylammonium bromide mixed micellar system. Mathematical Programing, 89(1), p. 149-185 (2000). Read more about how to correctly acknowledge RSC content. 2009 Oct 8;52(19):5896-905. doi: 10.1021/jm900282z. Epub 2010 Sep 20. Subscripts Critical micelle concentrations of the sodium salts. c, cyl
Radius of the spherical part of the micelle (m)
 <>
(3), Stay informed of issues for this journal through your RSS reader, Text Cylindrical. Langmuir, 18(01), p. 31-38 (2002). J Phys Chem B. If you want to reproduce the whole
<>
this article in other publications, without requesting further permission from the RSC,
The DeltaG(m) values progressively became less negative with the increase in the number of headgroups. The effects of inorganic salts (LiBr, NaBr, MgBr2), organic alcohols (C2H5OH, C3H7OH, C4H9OH, C5H11OH) and temperature on the critical micelle concentration (CMC) values of [Cnmpy][Br] aqueous solutions were also investigated. Langmuir, 28(3), p. 1738-1752 (2012). You can use material from
For [C14mpy][Br] and [C16mpy][Br] this transition occurs at 288.15 K and the micellization process is entropy-driven in the investigated temperature range. Accessibility Length of the cylindrical part of the micelle (m) Escola de Qumica, Universidade Federal do Rio de Janeiro, CEP: 21941-909, Rio de Janeiro - RJ, Brazil. Langmuir, 30(22), p. 6373-6383 (2014). Clearance Center request page.
Surfactant Langmuir, 25(20), p. 12101-12113 (2009). Bethesda, MD 20894, Web Policies D. Fu, X. Gao, B. Huang, J. Wang, Y. DOI: 10.1039/C9RA04226A. Sun, W. Zhang, K. Kan, X. Zhang, Y. Xie and X. Sui,
<>
(3), Stay informed of issues for this journal through your RSS reader, Text Cylindrical. Langmuir, 18(01), p. 31-38 (2002). J Phys Chem B. If you want to reproduce the whole
<>
this article in other publications, without requesting further permission from the RSC,
The DeltaG(m) values progressively became less negative with the increase in the number of headgroups. The effects of inorganic salts (LiBr, NaBr, MgBr2), organic alcohols (C2H5OH, C3H7OH, C4H9OH, C5H11OH) and temperature on the critical micelle concentration (CMC) values of [Cnmpy][Br] aqueous solutions were also investigated. Langmuir, 28(3), p. 1738-1752 (2012). You can use material from
For [C14mpy][Br] and [C16mpy][Br] this transition occurs at 288.15 K and the micellization process is entropy-driven in the investigated temperature range. Accessibility Length of the cylindrical part of the micelle (m) Escola de Qumica, Universidade Federal do Rio de Janeiro, CEP: 21941-909, Rio de Janeiro - RJ, Brazil. Langmuir, 30(22), p. 6373-6383 (2014). Clearance Center request page.
Surfactant Langmuir, 25(20), p. 12101-12113 (2009). Bethesda, MD 20894, Web Policies D. Fu, X. Gao, B. Huang, J. Wang, Y. DOI: 10.1039/C9RA04226A. Sun, W. Zhang, K. Kan, X. Zhang, Y. Xie and X. Sui,
Colloids and Surfaces A: Physicochemical and Engineering Aspects, 412, p. 90-95 (2012).
3 0 obj Complementary use of simulations and molecular-thermodynamic theory to model micellization. Part I. Conventional (pH-Insensitive) surfactants.
The CMC values assumed a trend of decreasing and then increasing with the increase of temperature. (English), https://doi.org/10.1590/0104-6632.20160333s20150129. Kamranfar, P. and Jamialahmadi M., Effect of surfactant micelle shape transition on the microemulsion viscosity and its application in enhanced oil recovery processes. These studies suggest that the micelles are more hydrated with multiheaded surfactants and the micropolarity of micelles increases with the increase in the number of headgroups. Neves, A. C. S., Valente, A. J. M., Burrows, H. D., Ribeiro, A. C. F., Lobo, V. M. M., Effect of terbium(III) chloride on the micellization properties of sodium decyl and dodecyl-sulfate solutions. Microcalorimetric and conductivity studies with micelles prepared from multi-headed pyridinium surfactants. w Number of molecules of i government site. <>/XObject<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI] >>/MediaBox[ 0 0 612 792] /Contents 4 0 R/Group<>/Tabs/S/StructParents 0>> Journal of Chemical Society, p. 579-586 (1956). P You do not have JavaScript enabled. E-mail: tavares@eq.ufrj.br. Free energy of micellization (J) endobj Kennedy, J. and Eberhart, R., Particle swarm optimization. Tiwary LK, Mandal A, Alam MS, Thennarasu S, Mandal AB. Creative Commons Attribution-NonCommercial 3.0 Unported Licence. and transmitted securely. Langmuir, 30(12), p. 3387-3396 (2014). Federal government websites often end in .gov or .mil. Negative DeltaH(m) values increased from CTAB to h = 1 to h = 2 and decreased for h = 3 whereas DeltaS(m) values decreased with increase in the number of headgroups. 8600 Rockville Pike Volume of the surfactant tail (m3) please go to the Copyright Clearance Center request page. Pf Packing factor J. Chem. The critical micellar concentrations (cmc's) and the degrees of counterion dissociation (alpha) of micelles of these surfactants were also determined by conductometry. Rs
This article is licensed under a
Velzquez, M. M. and Lpez-Daz, D., Variation of the critical micelle concentration with surfactant structure: A simple method to analyze the role of attractive - repulsive forces on micellar association. Journal of Chemical Society of Pakistan, 30(2), p. 186-191 (2008). Frenkel YV, Gallicchio E, Das K, Levy RM, Arnold E. J Med Chem. kankan.has@foxmail.com, xc_zhang.has@hotmail.com, b endobj