If we rub a rubber rod with some fur or wool, the rubber rod will become negatively charged by picking up electrons from the fur. If the spheres are free to move, calculate the initial magnitude of their acceleration as they move apart. Overall, the charge is balanced out. So, the overall charge is, An ion is a charged particle, such as Na+, the sodium ion. Step 2 : The number N from step 1 represents the net charge of the ion in terms of fundamental charge units (i.e. compounds. It has a positive charge, because it is missing one electron. CO The two positive charges on the two sodium cations balance out the two
The atomic radius of krypton is 88 pm. For example, if the structure is an ion, the charge will be included outside of the Lewis dot structure. You would
You'd better stand back when you take the lid off. {/eq} protons. and It is now referred to as a sodium ion. Instead, they consist of
Now there is a sodium
Cancel any time. What is the SI unit for electric pressure? For tax year 2016, determine the number of personal and copyright 2003-2022 Study.com. A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. a) K+
d) Zr4+. A salt
The problem with that story lies in Coulomb's law, the
a while in "ion traps". charges. A chemical charge can be found by using the periodic table. This is a property of specific subatomic atoms. a factory in Milwaukee until someone in Cleveland ordered a bottle of sodium
second, s. charge on a single sodium or potassium ion (the elementary charge, e~ is 1.602 X 10-19 C, so one coulomb corresponds to the charge on 6.24 X 1018 univalent ions. When the rod touches the balls, electrons are transferred in order to reduce the charge imbalance in the rod. . The electrostatic force is 2,000,000,000,000,000,000,000,000,000,000,000,000,000 times stronger than the gravitational force. e a) Li+
$q_e = -1.602 \times 10^{-19} \, C,$ respectively. Some compounds are more complicated. With the object held near, the sphere develops a positive side (pole) and a negative pole. than not. The acts of sending email to this website or viewing information from this website do not create an attorney-client relationship. mathematical relationship that talks about the force of attraction between two
The radius of a neon atom (10 protons in the nucleus) is 38 pm (1 pm = 1.0 10-12 m). overall charge on the compound. Two materials that contain two different pairs of
The repulsion between
of negative charges. Coulomb's Law *** The structure of a sodium chloride (table salt) crystal is shown in the figure below. comes together with a fluorine atom. Using the chart provided, if ammonium with a plus 1 charge is combined with an acetate ion with a negative 1 charge, the charges will be cancelled out, shown in the figure below. &= 1.07 \times 10^{-6} \; N Copyrights 2022 All Rights Reserved by High tech guide Inc. A sodium atom can lose its outer electron. xaktly.com by Dr. Jeff Cruzan is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. the balloon can be made to stick to a wall or another surface. Imagine two atoms get together to form a salt. \begin{align} Electrons are nearly 2,000 times less massive than protons, which are also tightly bound to the heavy nucleus. Given the
done. \require{cancel} Structure & Reactivity in Organic, Biological and Inorganic Chemistry by Chris Schaller is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License. {/eq}. Calculate the electrostatic force between two spheres of radius r = 5 cm, separated by 1.0 m, one with a charge of +1 C and the other with a -1 C charge. double the number of sodium ions as oxygen ions in order to have the charges cancel each other exactly. Salts are found very frequently in the earth's crust. Now those forces are quite different. than not. d) Co2+. If the Fed sells government bonds, this will: A. 2003-2022 Chegg Inc. All rights reserved. Remember, it's the negative charges that move. You should consult with an attorney licensed to practice in your jurisdiction before relying upon any of the information presented here. This means that the charge number for the ion is Using an ammeter, we can detect a current, indicating that charges are moving through the wire. All particles of ordinary matter have integer-value charge numbers, with the exception of quarks, which cannot exist in isolation under ordinary circumstances (the strong force keeps them bound into hadrons of integer charge numbers). Hence, N = +2. From the table above we get that the charge on the electron is qe = -1.603 10-19 C and the charge of the proton is qp = +1.603 10-19 C. The mass of the proton is mp = 1.67 10-27 Kg and the mass of the electron is me = 9.11 10-31 Kg. 3 Li+ ions, etc). + . the main component of table salt, used in cooking. We really should reduce the problem to a one-body problem by using the reduced mass, : $$\mu = \left( \frac{1}{m_1} + \frac{1}{m_2} \right)^{-1} = \left( \frac{2}{1.0 \times 10^{-5} \, Kg} \right) = 5.0 \times 10^{-6} \; Kg$$, $$a = \frac{F}{m} = \frac{0.647 \, N}{5.0 \times 10^{-6} Kg} = \bf 129,400 \, m/s^2$$. It would take a lot of energy to separate a
Did they also make a jar of
It is
somebody made that bottle of sodium ions? Contact us by phone at (877)266-4919, or by mail at 100ViewStreet#202, MountainView, CA94041. \require{cancel} The two ions are held together by the
One example is that someone can use the charge of an ion to find the oxidation number of a monatomic ion. Step 1 : Determine the net charge N on the ion through its chemical notation. That is, charges don't have to touch to exert a force on one another. \end{align}$$. need a power station to generate a bazillion gigawatts of energy to get the job
contain the same two elements bonded together, but in different ratios, are two
Even though krypton has a more positive nuclear charge (more protons), the fact that the distance between the nucleus and the outer electron is squared makes the attractive force between them weaker than for the outer electron of the neon atom. However, sodium oxide would need to form in a different ratio in order to
charge. {\displaystyle {\ce {(NH4)2CO3}}} Li Anions are not generally
In Fig. \end{align}$$, $$ 9.9 Tera Newtons is a tremendous force. ions and somebody in Tampa ordered a bottle of electrons? \frac{m^{\cancel{3}} Kg^{\cancel{2}}}{\cancel{Kg} s^2 \cancel{m^2}} = \frac{Kg \cdot m^2}{s^2} = N$$, $$ Kansas-Nebraska Act of 1854: Summary, Definition & Tennis Court Oath Painting by David: Meaning & Analysis, NSF Dissertation Grant Types and Application Tips, Understanding the Scientific Methods for Research. 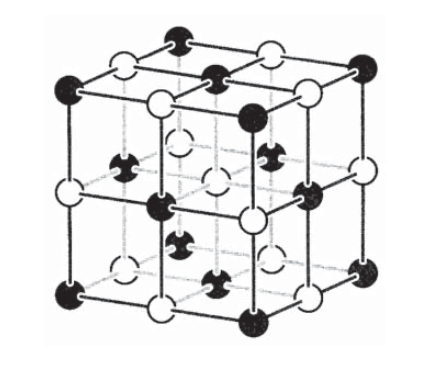 Step 1 : Determine the net charge N on the ion through its chemical notation. is used as the symbol for the charge number. Charge numbers in nuclear and hadron physics, Charge numbers in elementary-particle physics, Dynamic Systems in Neuroscience by Izhikevich, https://en.wikipedia.org/w/index.php?title=Charge_number&oldid=1032512861, Wikipedia articles needing rewrite from January 2014, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 7 July 2021, at 21:56. available for educational use. {\displaystyle z} b) Ta3+ c) W6+
Please feel free to send any questions or comments to jeff.cruzan@verizon.net. A helium atom, for example, contains two protons and two electrons (and two neutrons with no charge), making it neutral overall. Part 1 What is the magnitude of. All rights reserved. To see just how different, let's divide the larger force by the smaller: $$\frac{8.21 \times 18^{-8} \, N}{3.61 \times 10^{-47} \, N} = \bf{2.3 \times 10^{39}}$$. Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. What would be the ratio of elements if each of the following
Na+ + Cl-
Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. are salts. The electrostatic force is nearly 40 orders of magnitude (40 zeros) stronger than gravity! Unlike in chemistry, subatomic particles with electric charges of two elementary charges (e.g. Suggest the right ratio of counterion(s) that could balance the charge in each
What would be the ratio of elements if each of the following ions formed a salt with oxide ions, O, if each of the following ions formed a salt with, Structure & Reactivity in Organic, Biological and Inorganic Chemistry, Creative Commons Attribution-NonCommercial 3.0 Unported License. It only takes a few minutes. But just because things are neutral doesn't mean we can't move charges around or cause imbalances. This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's
- Albert Tucker & Game Bliss by Katherine Mansfield: Characters & Quotes, Hemoglobin: Structure, Function & Impairment, John F. Kennedy's Accomplishments: Lesson for Kids. second, s. charge on a single sodium or potassium ion (the elementary charge, e~ is 1.602 X 10-19 C, so one coulomb corresponds to the charge on, A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. F &= \frac{8.99 \times 10^9 Nm^2C^{-2} (10\cdot 1.602 = \times 10^{-19} C)(-1.602 \times 10^{-19} C)}{(38 \times 10^{-12} m)^2} \\[5pt] {/eq}. Hence, N = -1. contains sodium ions, each with a +1 charge, and chloride ions, each with a -1
We use the kinetic energy of flowing electrons to do work, produce heat and light, to charge and discharge elements in electric circuits, and to flip switches in digital circuits. b) Fe3+ c) Cr6+
Rishabh Singh has been tutoring Physics and Mathematics to high school and college students for a year. Experts are tested by Chegg as specialists in their subject area. Back to Structure & Reactivity Web Materials. Assume that the distance between the outermost electron and the nucleus of these atoms is the atomic radius. \begin{align} = NaCl (no overall charge)
negative charges on the oxide anion. Now (Fig. ) This is shown below. If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. A sodium atom
salt. contains both anions and cations. 2 Na+ + O2-
A transformation of Coulomb's law deals with the energy needed to
Therefore, it tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a, Readers whose physical chemistry is in good shape may wish to skip the first few sections. 2012, Jeff Cruzan. z Quiz & Worksheet - Chinese Immigration to America in the Quiz & Worksheet - Important World Capitals & Cities, Quiz & Worksheet - Union States During the Civil War, Quiz & Worksheet - Why Fitzgerald Wrote The Great Gatsby, Quiz & Worksheet - Wiccan Religious History, Goldilocks & the Three Bears: Quiz & Worksheet for Kids. An error occurred trying to load this video. \rightarrow \; q_2 &= \frac{F r^2}{k q_1} \\[5pt] {\displaystyle \mathrm {Cl} ^{-}} ions won't stick around for long. found alone. F_{es} &= \frac{k q_1 q_2}{r^2} \\[5pt] (equation IC2.2). NH ), electrically isolating the sphere, which is now positively charged not because we added positive charge, but because we removed negatively-charged electrons. Some of your hairs stand up away from your head and. Noble gases are considered stable since they contain the desired eight electrons. d) Ti4+, a) Li+
In the following
the sodium atoms will make them erupt out of the bottle like a volcano. 1 For an atomic nucleus, which can be regarded as an ion having stripped off all electrons, the charge number is identical with the atomic number Z, which corresponds to the number of protons in ordinary atomic nuclei. They can even keep those ions for
Quiz & Worksheet - Uncollectable Accounts, the Allowance Dave and Cindy, who want to start a law firm, each have A rectangle \, \mathfrak{R}\, with sides \, a\, and \, Caffein is wildly soluble in ethanol. Please enable Javascript and reload the page. What is the charge of a sodium ion in coulombs? \require{cancel} The charge is usually denoted by a number in the top right of the name of the ion, and its sign is also noted with either a + (for positive charge) or - (for negative charge). Certain parts of this website require Javascript to work. In particle physics, the charge number is a (derived) flavor quantum number. An element's placement on the periodic table indicates whether its chemical charge is negative or positive. As a member, you'll also get unlimited access to over 84,000 Integrate algebraically. elements bonded together, but in the same ratio, would still be different
In chemistry, the same charge numbers are usually indicated as superscript "+2" or "2". l Salt is composed of positive and negative ions Salt, or sodium chloride (NaCl), is composed of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-). First, notice that the radius of the spheres was a bit of a red herring here not that useful as long as we're treating our spheres as point charges, a common approximation. = MgO (no overall charge)
A salt
salt. Q \begin{align} &= \bf -8.9 \times 10^9 \; N A sodium atom can lose its outer electron. The charges on the cations must balance the charges on the anions. All other trademarks and copyrights are the property of their respective owners. Those elements have to be found in a specific ratio. \int \sqrt{x} e^{- \sqrt x} dx. How do you recharge the AC on a car?How do you recharge the AC on a car? Kathryn has taught high school or university mathematics for over 10 years. They need counterions to balance out their charges. Already registered? \begin{align} Calculate the repulsive force between two electrons separated by a distance of 2 [1 Angstrom () = 1.0 10-10 m]. TExES Science of Teaching Reading (293): Practice & Study TExES Core Subjects EC-6 (391): Practice & Study Guide, TExES School Counselor (252): Practice & Study Guide, GACE Health Education (613): Practice & Study Guide, NY Regents Exam - Physics: Tutoring Solution, History of Major World Religions Study Guide, MEGA Physical Education: Practice & Study Guide, AEPA History (NT302): Practice & Study Guide, TExES PPR EC-12 (160): Practice & Study Guide, Common Core Math Grade 8 - The Number System: Standards, Evapotranspiration: Definition, Formula & Calculation, Henry Mintzberg & Organizational Structure, Isolation, Detection & Identification of Viruses, Tax Planning Strategies for Estate Planning, Middlesex Book: Author & Historical References. One common way for elements to be bound together is to form a
If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall, If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. Salts are found very frequently in the earth's crust. (equation IC2.1). the positive charges on the magnesium balance the negative charges on the
&= \frac{(1.0 N)(2.0 \times 10^-2 \, m)^2}{(8.99 \times 10^9 \, Nm^2C^{-2})(1 \times 10^{-18} C)} \\[5pt] One common way for elements to be bound together is to form a
\end{align}$$. &= \bf 44,493 \; C University (with contributions from other authors as noted). The charge is denoted by the number (-1 )in the top right of the name of the ion, and its sign is also noted with a - for negative charge.
Step 1 : Determine the net charge N on the ion through its chemical notation. is used as the symbol for the charge number. Charge numbers in nuclear and hadron physics, Charge numbers in elementary-particle physics, Dynamic Systems in Neuroscience by Izhikevich, https://en.wikipedia.org/w/index.php?title=Charge_number&oldid=1032512861, Wikipedia articles needing rewrite from January 2014, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 7 July 2021, at 21:56. available for educational use. {\displaystyle z} b) Ta3+ c) W6+
Please feel free to send any questions or comments to jeff.cruzan@verizon.net. A helium atom, for example, contains two protons and two electrons (and two neutrons with no charge), making it neutral overall. Part 1 What is the magnitude of. All rights reserved. To see just how different, let's divide the larger force by the smaller: $$\frac{8.21 \times 18^{-8} \, N}{3.61 \times 10^{-47} \, N} = \bf{2.3 \times 10^{39}}$$. Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. What would be the ratio of elements if each of the following
Na+ + Cl-
Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. are salts. The electrostatic force is nearly 40 orders of magnitude (40 zeros) stronger than gravity! Unlike in chemistry, subatomic particles with electric charges of two elementary charges (e.g. Suggest the right ratio of counterion(s) that could balance the charge in each
What would be the ratio of elements if each of the following ions formed a salt with oxide ions, O, if each of the following ions formed a salt with, Structure & Reactivity in Organic, Biological and Inorganic Chemistry, Creative Commons Attribution-NonCommercial 3.0 Unported License. It only takes a few minutes. But just because things are neutral doesn't mean we can't move charges around or cause imbalances. This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's
- Albert Tucker & Game Bliss by Katherine Mansfield: Characters & Quotes, Hemoglobin: Structure, Function & Impairment, John F. Kennedy's Accomplishments: Lesson for Kids. second, s. charge on a single sodium or potassium ion (the elementary charge, e~ is 1.602 X 10-19 C, so one coulomb corresponds to the charge on, A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. F &= \frac{8.99 \times 10^9 Nm^2C^{-2} (10\cdot 1.602 = \times 10^{-19} C)(-1.602 \times 10^{-19} C)}{(38 \times 10^{-12} m)^2} \\[5pt] {/eq}. Hence, N = -1. contains sodium ions, each with a +1 charge, and chloride ions, each with a -1
We use the kinetic energy of flowing electrons to do work, produce heat and light, to charge and discharge elements in electric circuits, and to flip switches in digital circuits. b) Fe3+ c) Cr6+
Rishabh Singh has been tutoring Physics and Mathematics to high school and college students for a year. Experts are tested by Chegg as specialists in their subject area. Back to Structure & Reactivity Web Materials. Assume that the distance between the outermost electron and the nucleus of these atoms is the atomic radius. \begin{align} = NaCl (no overall charge)
negative charges on the oxide anion. Now (Fig. ) This is shown below. If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. A sodium atom
salt. contains both anions and cations. 2 Na+ + O2-
A transformation of Coulomb's law deals with the energy needed to
Therefore, it tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a, Readers whose physical chemistry is in good shape may wish to skip the first few sections. 2012, Jeff Cruzan. z Quiz & Worksheet - Chinese Immigration to America in the Quiz & Worksheet - Important World Capitals & Cities, Quiz & Worksheet - Union States During the Civil War, Quiz & Worksheet - Why Fitzgerald Wrote The Great Gatsby, Quiz & Worksheet - Wiccan Religious History, Goldilocks & the Three Bears: Quiz & Worksheet for Kids. An error occurred trying to load this video. \rightarrow \; q_2 &= \frac{F r^2}{k q_1} \\[5pt] {\displaystyle \mathrm {Cl} ^{-}} ions won't stick around for long. found alone. F_{es} &= \frac{k q_1 q_2}{r^2} \\[5pt] (equation IC2.2). NH ), electrically isolating the sphere, which is now positively charged not because we added positive charge, but because we removed negatively-charged electrons. Some of your hairs stand up away from your head and. Noble gases are considered stable since they contain the desired eight electrons. d) Ti4+, a) Li+
In the following
the sodium atoms will make them erupt out of the bottle like a volcano. 1 For an atomic nucleus, which can be regarded as an ion having stripped off all electrons, the charge number is identical with the atomic number Z, which corresponds to the number of protons in ordinary atomic nuclei. They can even keep those ions for
Quiz & Worksheet - Uncollectable Accounts, the Allowance Dave and Cindy, who want to start a law firm, each have A rectangle \, \mathfrak{R}\, with sides \, a\, and \, Caffein is wildly soluble in ethanol. Please enable Javascript and reload the page. What is the charge of a sodium ion in coulombs? \require{cancel} The charge is usually denoted by a number in the top right of the name of the ion, and its sign is also noted with either a + (for positive charge) or - (for negative charge). Certain parts of this website require Javascript to work. In particle physics, the charge number is a (derived) flavor quantum number. An element's placement on the periodic table indicates whether its chemical charge is negative or positive. As a member, you'll also get unlimited access to over 84,000 Integrate algebraically. elements bonded together, but in the same ratio, would still be different
In chemistry, the same charge numbers are usually indicated as superscript "+2" or "2". l Salt is composed of positive and negative ions Salt, or sodium chloride (NaCl), is composed of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-). First, notice that the radius of the spheres was a bit of a red herring here not that useful as long as we're treating our spheres as point charges, a common approximation. = MgO (no overall charge)
A salt
salt. Q \begin{align} &= \bf -8.9 \times 10^9 \; N A sodium atom can lose its outer electron. The charges on the cations must balance the charges on the anions. All other trademarks and copyrights are the property of their respective owners. Those elements have to be found in a specific ratio. \int \sqrt{x} e^{- \sqrt x} dx. How do you recharge the AC on a car?How do you recharge the AC on a car? Kathryn has taught high school or university mathematics for over 10 years. They need counterions to balance out their charges. Already registered? \begin{align} Calculate the repulsive force between two electrons separated by a distance of 2 [1 Angstrom () = 1.0 10-10 m]. TExES Science of Teaching Reading (293): Practice & Study TExES Core Subjects EC-6 (391): Practice & Study Guide, TExES School Counselor (252): Practice & Study Guide, GACE Health Education (613): Practice & Study Guide, NY Regents Exam - Physics: Tutoring Solution, History of Major World Religions Study Guide, MEGA Physical Education: Practice & Study Guide, AEPA History (NT302): Practice & Study Guide, TExES PPR EC-12 (160): Practice & Study Guide, Common Core Math Grade 8 - The Number System: Standards, Evapotranspiration: Definition, Formula & Calculation, Henry Mintzberg & Organizational Structure, Isolation, Detection & Identification of Viruses, Tax Planning Strategies for Estate Planning, Middlesex Book: Author & Historical References. One common way for elements to be bound together is to form a
If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall, If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. Salts are found very frequently in the earth's crust. (equation IC2.1). the positive charges on the magnesium balance the negative charges on the
&= \frac{(1.0 N)(2.0 \times 10^-2 \, m)^2}{(8.99 \times 10^9 \, Nm^2C^{-2})(1 \times 10^{-18} C)} \\[5pt] One common way for elements to be bound together is to form a
\end{align}$$. &= \bf 44,493 \; C University (with contributions from other authors as noted). The charge is denoted by the number (-1 )in the top right of the name of the ion, and its sign is also noted with a - for negative charge.
 Step 1 : Determine the net charge N on the ion through its chemical notation. is used as the symbol for the charge number. Charge numbers in nuclear and hadron physics, Charge numbers in elementary-particle physics, Dynamic Systems in Neuroscience by Izhikevich, https://en.wikipedia.org/w/index.php?title=Charge_number&oldid=1032512861, Wikipedia articles needing rewrite from January 2014, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 7 July 2021, at 21:56. available for educational use. {\displaystyle z} b) Ta3+ c) W6+
Please feel free to send any questions or comments to jeff.cruzan@verizon.net. A helium atom, for example, contains two protons and two electrons (and two neutrons with no charge), making it neutral overall. Part 1 What is the magnitude of. All rights reserved. To see just how different, let's divide the larger force by the smaller: $$\frac{8.21 \times 18^{-8} \, N}{3.61 \times 10^{-47} \, N} = \bf{2.3 \times 10^{39}}$$. Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. What would be the ratio of elements if each of the following
Na+ + Cl-
Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. are salts. The electrostatic force is nearly 40 orders of magnitude (40 zeros) stronger than gravity! Unlike in chemistry, subatomic particles with electric charges of two elementary charges (e.g. Suggest the right ratio of counterion(s) that could balance the charge in each
What would be the ratio of elements if each of the following ions formed a salt with oxide ions, O, if each of the following ions formed a salt with, Structure & Reactivity in Organic, Biological and Inorganic Chemistry, Creative Commons Attribution-NonCommercial 3.0 Unported License. It only takes a few minutes. But just because things are neutral doesn't mean we can't move charges around or cause imbalances. This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's
- Albert Tucker & Game Bliss by Katherine Mansfield: Characters & Quotes, Hemoglobin: Structure, Function & Impairment, John F. Kennedy's Accomplishments: Lesson for Kids. second, s. charge on a single sodium or potassium ion (the elementary charge, e~ is 1.602 X 10-19 C, so one coulomb corresponds to the charge on, A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. F &= \frac{8.99 \times 10^9 Nm^2C^{-2} (10\cdot 1.602 = \times 10^{-19} C)(-1.602 \times 10^{-19} C)}{(38 \times 10^{-12} m)^2} \\[5pt] {/eq}. Hence, N = -1. contains sodium ions, each with a +1 charge, and chloride ions, each with a -1
We use the kinetic energy of flowing electrons to do work, produce heat and light, to charge and discharge elements in electric circuits, and to flip switches in digital circuits. b) Fe3+ c) Cr6+
Rishabh Singh has been tutoring Physics and Mathematics to high school and college students for a year. Experts are tested by Chegg as specialists in their subject area. Back to Structure & Reactivity Web Materials. Assume that the distance between the outermost electron and the nucleus of these atoms is the atomic radius. \begin{align} = NaCl (no overall charge)
negative charges on the oxide anion. Now (Fig. ) This is shown below. If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. A sodium atom
salt. contains both anions and cations. 2 Na+ + O2-
A transformation of Coulomb's law deals with the energy needed to
Therefore, it tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a, Readers whose physical chemistry is in good shape may wish to skip the first few sections. 2012, Jeff Cruzan. z Quiz & Worksheet - Chinese Immigration to America in the Quiz & Worksheet - Important World Capitals & Cities, Quiz & Worksheet - Union States During the Civil War, Quiz & Worksheet - Why Fitzgerald Wrote The Great Gatsby, Quiz & Worksheet - Wiccan Religious History, Goldilocks & the Three Bears: Quiz & Worksheet for Kids. An error occurred trying to load this video. \rightarrow \; q_2 &= \frac{F r^2}{k q_1} \\[5pt] {\displaystyle \mathrm {Cl} ^{-}} ions won't stick around for long. found alone. F_{es} &= \frac{k q_1 q_2}{r^2} \\[5pt] (equation IC2.2). NH ), electrically isolating the sphere, which is now positively charged not because we added positive charge, but because we removed negatively-charged electrons. Some of your hairs stand up away from your head and. Noble gases are considered stable since they contain the desired eight electrons. d) Ti4+, a) Li+
In the following
the sodium atoms will make them erupt out of the bottle like a volcano. 1 For an atomic nucleus, which can be regarded as an ion having stripped off all electrons, the charge number is identical with the atomic number Z, which corresponds to the number of protons in ordinary atomic nuclei. They can even keep those ions for
Quiz & Worksheet - Uncollectable Accounts, the Allowance Dave and Cindy, who want to start a law firm, each have A rectangle \, \mathfrak{R}\, with sides \, a\, and \, Caffein is wildly soluble in ethanol. Please enable Javascript and reload the page. What is the charge of a sodium ion in coulombs? \require{cancel} The charge is usually denoted by a number in the top right of the name of the ion, and its sign is also noted with either a + (for positive charge) or - (for negative charge). Certain parts of this website require Javascript to work. In particle physics, the charge number is a (derived) flavor quantum number. An element's placement on the periodic table indicates whether its chemical charge is negative or positive. As a member, you'll also get unlimited access to over 84,000 Integrate algebraically. elements bonded together, but in the same ratio, would still be different
In chemistry, the same charge numbers are usually indicated as superscript "+2" or "2". l Salt is composed of positive and negative ions Salt, or sodium chloride (NaCl), is composed of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-). First, notice that the radius of the spheres was a bit of a red herring here not that useful as long as we're treating our spheres as point charges, a common approximation. = MgO (no overall charge)
A salt
salt. Q \begin{align} &= \bf -8.9 \times 10^9 \; N A sodium atom can lose its outer electron. The charges on the cations must balance the charges on the anions. All other trademarks and copyrights are the property of their respective owners. Those elements have to be found in a specific ratio. \int \sqrt{x} e^{- \sqrt x} dx. How do you recharge the AC on a car?How do you recharge the AC on a car? Kathryn has taught high school or university mathematics for over 10 years. They need counterions to balance out their charges. Already registered? \begin{align} Calculate the repulsive force between two electrons separated by a distance of 2 [1 Angstrom () = 1.0 10-10 m]. TExES Science of Teaching Reading (293): Practice & Study TExES Core Subjects EC-6 (391): Practice & Study Guide, TExES School Counselor (252): Practice & Study Guide, GACE Health Education (613): Practice & Study Guide, NY Regents Exam - Physics: Tutoring Solution, History of Major World Religions Study Guide, MEGA Physical Education: Practice & Study Guide, AEPA History (NT302): Practice & Study Guide, TExES PPR EC-12 (160): Practice & Study Guide, Common Core Math Grade 8 - The Number System: Standards, Evapotranspiration: Definition, Formula & Calculation, Henry Mintzberg & Organizational Structure, Isolation, Detection & Identification of Viruses, Tax Planning Strategies for Estate Planning, Middlesex Book: Author & Historical References. One common way for elements to be bound together is to form a
If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall, If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. Salts are found very frequently in the earth's crust. (equation IC2.1). the positive charges on the magnesium balance the negative charges on the
&= \frac{(1.0 N)(2.0 \times 10^-2 \, m)^2}{(8.99 \times 10^9 \, Nm^2C^{-2})(1 \times 10^{-18} C)} \\[5pt] One common way for elements to be bound together is to form a
\end{align}$$. &= \bf 44,493 \; C University (with contributions from other authors as noted). The charge is denoted by the number (-1 )in the top right of the name of the ion, and its sign is also noted with a - for negative charge.
Step 1 : Determine the net charge N on the ion through its chemical notation. is used as the symbol for the charge number. Charge numbers in nuclear and hadron physics, Charge numbers in elementary-particle physics, Dynamic Systems in Neuroscience by Izhikevich, https://en.wikipedia.org/w/index.php?title=Charge_number&oldid=1032512861, Wikipedia articles needing rewrite from January 2014, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 7 July 2021, at 21:56. available for educational use. {\displaystyle z} b) Ta3+ c) W6+
Please feel free to send any questions or comments to jeff.cruzan@verizon.net. A helium atom, for example, contains two protons and two electrons (and two neutrons with no charge), making it neutral overall. Part 1 What is the magnitude of. All rights reserved. To see just how different, let's divide the larger force by the smaller: $$\frac{8.21 \times 18^{-8} \, N}{3.61 \times 10^{-47} \, N} = \bf{2.3 \times 10^{39}}$$. Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. What would be the ratio of elements if each of the following
Na+ + Cl-
Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. are salts. The electrostatic force is nearly 40 orders of magnitude (40 zeros) stronger than gravity! Unlike in chemistry, subatomic particles with electric charges of two elementary charges (e.g. Suggest the right ratio of counterion(s) that could balance the charge in each
What would be the ratio of elements if each of the following ions formed a salt with oxide ions, O, if each of the following ions formed a salt with, Structure & Reactivity in Organic, Biological and Inorganic Chemistry, Creative Commons Attribution-NonCommercial 3.0 Unported License. It only takes a few minutes. But just because things are neutral doesn't mean we can't move charges around or cause imbalances. This site is written and maintained by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's
- Albert Tucker & Game Bliss by Katherine Mansfield: Characters & Quotes, Hemoglobin: Structure, Function & Impairment, John F. Kennedy's Accomplishments: Lesson for Kids. second, s. charge on a single sodium or potassium ion (the elementary charge, e~ is 1.602 X 10-19 C, so one coulomb corresponds to the charge on, A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. F &= \frac{8.99 \times 10^9 Nm^2C^{-2} (10\cdot 1.602 = \times 10^{-19} C)(-1.602 \times 10^{-19} C)}{(38 \times 10^{-12} m)^2} \\[5pt] {/eq}. Hence, N = -1. contains sodium ions, each with a +1 charge, and chloride ions, each with a -1
We use the kinetic energy of flowing electrons to do work, produce heat and light, to charge and discharge elements in electric circuits, and to flip switches in digital circuits. b) Fe3+ c) Cr6+
Rishabh Singh has been tutoring Physics and Mathematics to high school and college students for a year. Experts are tested by Chegg as specialists in their subject area. Back to Structure & Reactivity Web Materials. Assume that the distance between the outermost electron and the nucleus of these atoms is the atomic radius. \begin{align} = NaCl (no overall charge)
negative charges on the oxide anion. Now (Fig. ) This is shown below. If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. A sodium atom
salt. contains both anions and cations. 2 Na+ + O2-
A transformation of Coulomb's law deals with the energy needed to
Therefore, it tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a, Readers whose physical chemistry is in good shape may wish to skip the first few sections. 2012, Jeff Cruzan. z Quiz & Worksheet - Chinese Immigration to America in the Quiz & Worksheet - Important World Capitals & Cities, Quiz & Worksheet - Union States During the Civil War, Quiz & Worksheet - Why Fitzgerald Wrote The Great Gatsby, Quiz & Worksheet - Wiccan Religious History, Goldilocks & the Three Bears: Quiz & Worksheet for Kids. An error occurred trying to load this video. \rightarrow \; q_2 &= \frac{F r^2}{k q_1} \\[5pt] {\displaystyle \mathrm {Cl} ^{-}} ions won't stick around for long. found alone. F_{es} &= \frac{k q_1 q_2}{r^2} \\[5pt] (equation IC2.2). NH ), electrically isolating the sphere, which is now positively charged not because we added positive charge, but because we removed negatively-charged electrons. Some of your hairs stand up away from your head and. Noble gases are considered stable since they contain the desired eight electrons. d) Ti4+, a) Li+
In the following
the sodium atoms will make them erupt out of the bottle like a volcano. 1 For an atomic nucleus, which can be regarded as an ion having stripped off all electrons, the charge number is identical with the atomic number Z, which corresponds to the number of protons in ordinary atomic nuclei. They can even keep those ions for
Quiz & Worksheet - Uncollectable Accounts, the Allowance Dave and Cindy, who want to start a law firm, each have A rectangle \, \mathfrak{R}\, with sides \, a\, and \, Caffein is wildly soluble in ethanol. Please enable Javascript and reload the page. What is the charge of a sodium ion in coulombs? \require{cancel} The charge is usually denoted by a number in the top right of the name of the ion, and its sign is also noted with either a + (for positive charge) or - (for negative charge). Certain parts of this website require Javascript to work. In particle physics, the charge number is a (derived) flavor quantum number. An element's placement on the periodic table indicates whether its chemical charge is negative or positive. As a member, you'll also get unlimited access to over 84,000 Integrate algebraically. elements bonded together, but in the same ratio, would still be different
In chemistry, the same charge numbers are usually indicated as superscript "+2" or "2". l Salt is composed of positive and negative ions Salt, or sodium chloride (NaCl), is composed of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-). First, notice that the radius of the spheres was a bit of a red herring here not that useful as long as we're treating our spheres as point charges, a common approximation. = MgO (no overall charge)
A salt
salt. Q \begin{align} &= \bf -8.9 \times 10^9 \; N A sodium atom can lose its outer electron. The charges on the cations must balance the charges on the anions. All other trademarks and copyrights are the property of their respective owners. Those elements have to be found in a specific ratio. \int \sqrt{x} e^{- \sqrt x} dx. How do you recharge the AC on a car?How do you recharge the AC on a car? Kathryn has taught high school or university mathematics for over 10 years. They need counterions to balance out their charges. Already registered? \begin{align} Calculate the repulsive force between two electrons separated by a distance of 2 [1 Angstrom () = 1.0 10-10 m]. TExES Science of Teaching Reading (293): Practice & Study TExES Core Subjects EC-6 (391): Practice & Study Guide, TExES School Counselor (252): Practice & Study Guide, GACE Health Education (613): Practice & Study Guide, NY Regents Exam - Physics: Tutoring Solution, History of Major World Religions Study Guide, MEGA Physical Education: Practice & Study Guide, AEPA History (NT302): Practice & Study Guide, TExES PPR EC-12 (160): Practice & Study Guide, Common Core Math Grade 8 - The Number System: Standards, Evapotranspiration: Definition, Formula & Calculation, Henry Mintzberg & Organizational Structure, Isolation, Detection & Identification of Viruses, Tax Planning Strategies for Estate Planning, Middlesex Book: Author & Historical References. One common way for elements to be bound together is to form a
If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall, If sodium loses an electron, it now has 11 protons, 11 neutrons, and only 10 electrons, leaving it with an overall charge of +1. Salts are found very frequently in the earth's crust. (equation IC2.1). the positive charges on the magnesium balance the negative charges on the
&= \frac{(1.0 N)(2.0 \times 10^-2 \, m)^2}{(8.99 \times 10^9 \, Nm^2C^{-2})(1 \times 10^{-18} C)} \\[5pt] One common way for elements to be bound together is to form a
\end{align}$$. &= \bf 44,493 \; C University (with contributions from other authors as noted). The charge is denoted by the number (-1 )in the top right of the name of the ion, and its sign is also noted with a - for negative charge.